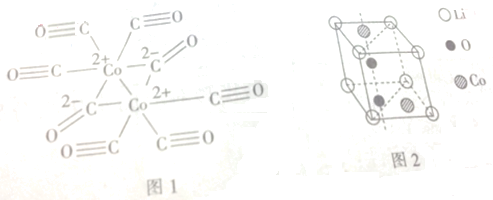
��Ŀ����
1�� ��֪A��B��C��D��E��F��G��H��IΪԪ�����ڱ���ԭ���������������ǰ������Ԫ�أ�Aԭ�ӵ��������p����ϵĵ���������ǰһ���Ӳ����������A��B��C��D��E��F��G�ֱ�λ��ͬһ���ڣ�Cԭ��L������2�Գɵ��ӣ�D��E��F�ĺ�������Ų���ͬ�ļ����ӿ��γ�һ��E3FD6�����Ӿ���X��EG��HCΪ��������ͬ�����Ӿ��壮Iԭ��M��Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ�������������������ش��������⣺������ʱ���ö�Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E��F��G��H��IΪԪ�����ڱ���ԭ���������������ǰ������Ԫ�أ�Aԭ�ӵ��������p����ϵĵ���������ǰһ���Ӳ����������A��B��C��D��E��F��G�ֱ�λ��ͬһ���ڣ�Cԭ��L������2�Գɵ��ӣ�D��E��F�ĺ�������Ų���ͬ�ļ����ӿ��γ�һ��E3FD6�����Ӿ���X��EG��HCΪ��������ͬ�����Ӿ��壮Iԭ��M��Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ�������������������ش��������⣺������ʱ���ö�Ӧ��Ԫ�ط��ű�ʾ����1����ѧ��ͨ��X����̽����MgO�ľ���ṹ��NaCl�ľ���ṹ���ƣ�MgO������һ��Mg2+��Χ�������ڽ��Ҿ�����ȵ�Mg2+��12�����������ڽ��Ҿ�����ȵ�O2-��6����MgO���۵㣾NaCl���۵㣨���������������������ԭ��MgO��NaClΪ���Ӿ��壬MgO������Mg2+��O2- ��������ɴ���NaCl������Na+��Cl-��������ɣ�Mg2+��O2- �İ뾶С��NaCl������Na+��Cl-�İ뾶��MgO�����ܴ�����MgO���۵��NaCl�ĸߣ�
��2��д��X�Ļ�ѧʽNa3AlF6
��3��д��X�漰���������е�һ����ѧ����ʽ2Al2O3�����ۣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$2Al+3 O2����
��4���Խ���ҵұ��F����FG3������F2C3Ϊԭ�ϵ�ԭ��Al2O3Ϊ���ӻ������AlCl3Ϊ���ۻ�������ۻ�����������״̬�²��ܵ���������ƶ������ӣ�������Al2O3Ϊԭ�϶�������AlCl3��
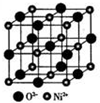
��5��H��D���γ����ӻ�����侧���ṹ��ͼ��ʾ��
H��D�γ����ӻ�����Ļ�ѧʽΪCaF2�������ӻ����ᄃ����ܶ�Ϊa g/cm3�����������$\frac{4��78g/mol}{ag/c{m}^{3}��{N}_{A}mo{l}^{-1}}$��ֻҪ���г���ʽ����
���� Aԭ�ӵ��������p����ϵĵ���������ǰһ���Ӳ����������ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����AΪ̼Ԫ�أ�Cԭ��L������2�ԳɶԵ��ӣ���������Ų�Ϊ1s2s22p4����CΪ��Ԫ�أ�A��B��C��Dԭ����������������ͬ���ڣ�����BΪ��Ԫ�أ���DΪ��Ԫ�أ�
D��E��F�ļ����Ӻ�������Ų���ͬ�����������Ϊ10����E��F���ڵ������ڣ����߿��γ�һ��E3FD6�����Ӿ���X����֪EΪ+1�ۡ�FΪ+3�ۣ���EΪNa��FΪAl��E��F��G�ֱ�λ��ͬһ���ڣ�EG��HCΪ��������ͬ�����Ӿ��壬��G����-1�ۡ�H����+2�ۣ�����֪GΪCl��HΪMg��Iԭ��M��Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ����ԭ�Ӻ��������Ϊ2+8+18+1=29����IΪCu���ݴ˽��
��� �⣺ԭ�ӵ��������p����ϵĵ���������ǰһ���Ӳ����������ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����AΪ̼Ԫ�أ�Cԭ��L������2�ԳɶԵ��ӣ���������Ų�Ϊ1s2s22p4����CΪ��Ԫ�أ�A��B��C��Dԭ����������������ͬ���ڣ�����BΪ��Ԫ�أ���DΪ��Ԫ�أ�
D��E��F�ļ����Ӻ�������Ų���ͬ�����������Ϊ10����E��F���ڵ������ڣ����߿��γ�һ��E3FD6�����Ӿ���X����֪EΪ+1�ۡ�FΪ+3�ۣ���EΪNa��FΪAl��E��F��G�ֱ�λ��ͬһ���ڣ�EG��HCΪ��������ͬ�����Ӿ��壬��G����-1�ۡ�H����+2�ۣ�����֪GΪCl��HΪMg��Iԭ��M��Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ����ԭ�Ӻ��������Ϊ2+8+18+1=29����IΪCu��
��1����ѧ��ͨ��X����̽����MgO�ľ���ṹ��NaCl�ľ���ṹ���ƣ������ṹΪ ���Զ���ΪMg2+�����о�����֮�����Mg2+����λ�����ģ�ÿ������Ϊ12���湲�ã���MgO������һ��Mg2+��Χ�������ڽ��Ҿ�����ȵ�Mg2+��12������֮���ڽ��Ҿ�����ȵ�O2-λ�����м䣬�ҹ���Mg2+�Գƣ���Mg2+���ڽ��Ҿ�����ȵ�O2-������6����
���Զ���ΪMg2+�����о�����֮�����Mg2+����λ�����ģ�ÿ������Ϊ12���湲�ã���MgO������һ��Mg2+��Χ�������ڽ��Ҿ�����ȵ�Mg2+��12������֮���ڽ��Ҿ�����ȵ�O2-λ�����м䣬�ҹ���Mg2+�Գƣ���Mg2+���ڽ��Ҿ�����ȵ�O2-������6����
MgO��NaClΪ���Ӿ��壬MgO������Mg2+��O2- ��������ɴ���NaCl������Na+��Cl-��������ɣ�Mg2+��O2- �İ뾶С��NaCl������Na+��Cl-�İ뾶��MgO�����ܴ�����MgO���۵��NaCl�ĸߣ�
�ʴ�Ϊ��12��6������MgO��NaClΪ���Ӿ��壬MgO������Mg2+��O2- ��������ɴ���NaCl������Na+��Cl-��������ɣ�Mg2+��O2- �İ뾶С��NaCl������Na+��Cl-�İ뾶��MgO�����ܴ�����MgO���۵��NaCl�ĸߣ�
��2��������������֪��X�Ļ�ѧʽΪNa3AlF6���ʴ�Ϊ��Na3AlF6��
��3��Na3AlF6�������ڹ�ҵұ��������Ӧ����ʽΪ��2Al2O3�����ۣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$2Al+3 O2�����ʴ�Ϊ��2Al2O3�����ۣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$2Al+3 O2����
��4��Al2O3Ϊ���ӻ������AlCl3Ϊ���ۻ�������ۻ�����������״̬�²��ܵ���������ƶ������ӣ�������Al2O3Ϊԭ�϶�������AlCl3��
�ʴ�Ϊ��Al2O3Ϊ���ӻ������AlCl3Ϊ���ۻ�������ۻ�����������״̬�²��ܵ���������ƶ������ӣ�������Al2O3Ϊԭ�϶�������AlCl3��
��5��������Caԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Fԭ����ĿΪ8���ʸ����ӻ����ﻯѧʽΪCaF2����������Ϊ4��$\frac{78g/mol}{{N}_{A}mo{l}^{-1}}$�������ӻ����ᄃ����ܶ�Ϊa g/cm3���������V=$\frac{4��78g/mol}{ag/c{m}^{3}��{N}_{A}mo{l}^{-1}}$��
�ʴ�Ϊ��CaF2��$\frac{4��78g/mol}{ag/c{m}^{3}��{N}_{A}mo{l}^{-1}}$��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����������������ʡ������ṹ������ȣ����ؿ��龧���ṹ����㣬ע�������ѧ�����ľ����ṹ���Ѷ��еȣ�

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| A�� | һ������Al��������Ϊ4.5g | |
| B�� | һ������FeCl2�����ܺ���MgCl2��AlCl3 | |
| C�� | һ������MgCl2��FeCl2 | |
| D�� | һ�����У�NH4��2SO4��MgCl2�������ʵ������ |
| A�� | H2SO4 | B�� | HCl | C�� | HClO | D�� | Ba��HSO3��2 |
| A�� | NH3�������ӻ����� | |
| B�� | �����£�Һ���ĵ���ƽ�ⳣ��Ϊ10-14 | |
| C�� | Һ̬���백ˮ�������ͬ | |
| D�� | Һ���е�������ͬ�ĵ����� |
| ѡ�� | A | B | C | D |
| n��H2SO4��/mol | 2 | 3 | 4 | 5 |
| n��������/mol | 2 | 3 | 2 | 1.5 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| IA | ��A | ��A | ��A | VA | ��A | VIIA | 0 | |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | ||
| 4 | �� | �� |
��2����������õĽ�����K�����ʳ�������Һ���Ԫ����Br��
��3���������γ��������������Ԫ����Al���ֱ�д����Ԫ�ص�����������ˮ������ޡ�������������ˮ���ﷴӦ�����ӷ���ʽ��Al��OH��3+3H+=Al3++3H2O��Al��OH��3+OH-=AlO2-+2H2O��
��4��д��һ�����ӷ���ʽ���ȽϢߡ��ⵥ�������Ե�ǿ����Cl2+2Br-=Br2+2Cl-��
 GTN{[Ni��CHZ��3]��ClO4��2}��һ�����͵���ҩ��
GTN{[Ni��CHZ��3]��ClO4��2}��һ�����͵���ҩ��
 +��CH3CO��2O��
+��CH3CO��2O��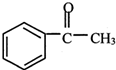 +CH3COOH
+CH3COOH �� B
�� B
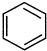 ֱ����ȡ
ֱ����ȡ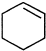 ��ԭ���DZ��е�̼̼����һ������ļ����������ӳɺ�ֻ�ܵû����飮
��ԭ���DZ��е�̼̼����һ������ļ����������ӳɺ�ֻ�ܵû����飮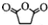 +
+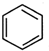 ��
��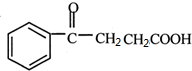 ��
��