��Ŀ����
16����1�������£�0.10mol/L NH4Cl ��Һ pH��7�����=����������Һ�и�����Ũ���ɴ�С��˳ ����c��Cl-����c��NH4+����c��H+����c��OH-������2����ͬ���ʵ���Ũ�ȵ� Na2S ��Һ�� NaHS ��Һ��pH ��С��Na2S��NaHS�����=���������� ����Һ�������ࣺNa2S=NaHS�����=��������
��3��NaHCO3 ��Һ�ʼ��Ե�ԭ����HCO3-+H2O?H2CO3+OH-��д���йص����ӷ���ʽ����ͬ����Al2��SO4��3��Һ�����Ե�ԭ����Al3++3H2O?Al��OH��3+3H+���� NaHCO3��Һ�� Al2��SO4��3 ��Һ��ϣ��������а�ɫ��������ɫ�����������ط�Ӧ�����ӷ���ʽ�ǣ�Al3++3HCO3-=Al��OH��3��+3CO2������ NaAlO2 ��NaHCO3��Һ��ϣ������ǰ�ɫ������������ �� �� Ӧ �� �� �� �� �� ʽ �ǣ�HCO3-+AlO2-+H2O=Al��OH��3��+CO32-��
���� ��1��NH4Cl��ǿ�������Σ�ˮ�������ԣ����ݵ���غ��ж�����Ũ�ȴ�С��
��2��Na2Sˮ��Һ�зֲ�ˮ�⣬�Ե�һ��ˮ��Ϊ����ͬʱ����HS-?H++S2-��
��3��NaHCO3Ϊǿ�������Σ�ˮ��ʼ��ԣ���������Һ��������ˮ������ԣ���������̼������ˮ��Һ�з���˫ˮ�������������������Ͷ�����̼��NaHCO3��Һ��NaAlO2��Һ��ϲ�����ɫ��������������
��� �⣺��1��笠�����ˮ�⣬ʹ��Һ�����ԣ���pH��7�������ӷ���ʽ��ʾΪNH4++H2O=NH3•H2O+H+��һ��������ε�ˮ��̶ȶ��Ƚ�С��������Һ������Ũ�ȴ�С˳��Ϊc��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ������c��Cl-����c��NH4+����c��H+����c��OH-����
��2��Na2S�Ե�һ��ˮ�⣬Na2S��ˮ��̶ȴ���NaHS������Na2S��PH����NaHS��ˮ�����ӷ���ʽΪ��S2-+H2O?HS-+OH-��HS-+H2O?H2S+OH-����Һ�л�����HS-?H++S2-������������Һ����������ͬ��
�ʴ�Ϊ������=��
��3��̼��������Һ�У�̼��������Ӳ���ˮ�⣺HCO3-+H2O?H2CO3+OH-������Һ��ʾ���ԣ���������Һ��������ˮ������ԣ���Ӧ�����ӷ���ʽΪ��Al3++3H2O?Al��OH��3+3H+����������̼������ˮ��Һ�з���˫ˮ�������������������Ͷ�����̼����Ӧ�����ӷ���ʽΪ��Al3++3HCO3-=Al��OH��3��+3CO2����NaHCO3��Һ��NaAlO2��Һ��ϲ�����ɫ��������������̼������ӣ���Ӧ���ӷ���ʽΪ��AlO2-+HCO3-+H2O�TAl��OH��3��+CO32-��
�ʴ�Ϊ��HCO3-+H2O?H2CO3+OH-��Al3++3H2O?Al��OH��3+3H+���а�ɫ��������ɫ���������Al3++3HCO3-=Al��OH��3��+3CO2�����а�ɫ����������HCO3-+AlO2-+H2O=Al��OH��3��+CO32-��
���� �����ǻ���������Ŀ��飬�����������У�������ǿ������Ĺؼ�����ȷ����ˮ���ԭ����Ȼ���������Ϣ������ü��ɣ�����������ѧ�������Ӧ������������ʱע�����ӷ�Ӧ����ʽ����ȷ��д��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�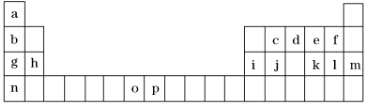
��1��Ԫ��pΪ26��Ԫ�أ���д�����̬ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d64s2��
��2��d��a��Ӧ�IJ���ķ����У�����ԭ�ӵ��ӻ���ʽΪsp3��
��3��h�ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ⣨�ӣ�����ʽ�ͷ�������
��4��o��p��Ԫ�صIJ��ֵ��������������±���
| Ԫ�� | o | p | |
| ������kJ•mol-1 | I1 | 717 | 759 |
| I2 | 1 509 | 1 561 | |
| I3 | 3 248 | 2 957 | |
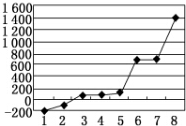
��5����������8��Ԫ�ذ������۵�ߵ͵�˳����ͼ��ʾ�����е縺��������2������ͼ�е���ţ���
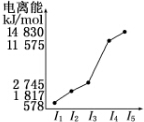
��6���������е�ij����Ԫ�صĵ����������ͼ��ʾ�����Ԫ����Al����Ԫ�ط��ţ���
| A�� | ��������Ũ�����Ũ������ | |
| B�� | ������������������Һ�� | |
| C�� | ���ǵ��۵㶼�ܸߣ��������ͻ���� | |
| D�� | �����¶�������ˮ��Ӧ |
| A�� | �����£�1L0.1mol•L-1��NH4NO3��Һ�е�ԭ����Ϊ0.2NA | |
| B�� | 1mol�ǻ��е�����Ϊ10 NA | |
| C�� | �ڷ�ӦKIO3+6HI�TKI+3I2+3H2O�У�ÿ����3 mol I2ת�Ƶĵ�����Ϊ6 NA | |
| D�� | ���³�ѹ�£�22.4L��ϩ��C--H����Ϊ4 NA |
| A�� | 100 L | B�� | 10 L | C�� | 11.2L | D�� | 44.8L |
| A�� | 5.6L | B�� | 11.2L | C�� | 22.4L | D�� | 33.6L |
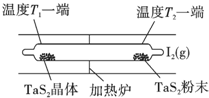 ��ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�ã�
��ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�ã�