��Ŀ����
ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽᣨ���ڳ����£���������ȷ����
�� pH��1��ǿ����Һ����ˮϡ�ͺ���Һ�и�����Ũ�ȶ��ή��
�� 1 L 0.50 mol��L��1NH4Cl ��Һ��2 L 0.25 mol��L��1NH4Cl ��Һ��NH4+ ���ʵ�����ȫ���
�� pH��ȵ�������Һ��a��CH3COONa b��C6H5ONa c��NaHCO3 d��NaOH����������Һ�����ʵ����ʵ���Ũ����С����˳��Ϊ��d < b < c < a
�� pH=8.3��NaHCO3��Һ��c(Na��) �� c(HCO3��) �� c(CO32��)�� c(H2CO3)
�� pH��2��һԪ���pH��12�Ķ�Ԫǿ��������ϣ�c(OH��) �� c(H��)
��pH��4��Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COOH��CH3COONa�����Һ�У�c(CH3COO��)��c(OH��) �� c(CH3COOH)��c(H+)
| A���٢ڢ� | B���٢ۢ� | C���ۢݢ� | D���ڢܢ� |
C
��������������� pH��1��ǿ����Һ����ˮϡ�ͺ���Һ�и�����Ũ�ȶ��ή�ͣ���������������Ũ�Ȼ����ߣ����� 1 L 0.50 mol��L��1NH4Cl ��Һ��2 L 0.25 mol��L��1NH4Cl ��Һ��NH4+ ���ʵ�����ȫ��ȣ����˵��Ҳ�Ǵ���ģ�ԭ����������Һ���Ȼ�淋�Ũ�Ȳ�һ����ˮ��̶Ȳ�һ������ pH��ȵ�������Һ��a��CH3COONa b��C6H5ONa c��NaHCO3 d��NaOH����������Һ�����ʵ����ʵ���Ũ����С����˳��Ϊ��d < b < c < a����ȷ����Ϊ����ǿ��˳��Ϊ�����ᡢ̼�ᡢ���ӣ�������Ӧ�����εļ���ǿ����Ϊ�����ơ�̼�����ơ������ƣ�������Һ�����ʵ����ʵ���Ũ����С�����˳��Ϊ��d < b < c < a���� pH=8.3��NaHCO3��Һ�У�̼��������Ӵ���ˮ��͵������������ƣ���������Һ�Լ��ԣ����ˮ��̶ȴ��ڵ���̶ȣ������У�c(Na��) �� c(HCO3��)�� c(H2CO3) �� c(CO32��)�����Դ��� pH��2��һԪ���pH��12�Ķ�Ԫǿ��������ϣ�c(OH��) �� c(H��)����ȷ����pH��4��Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COOH��CH3COONa�����Һ�У�������Һ�����ԣ�Ҳ����˵����ĵ���̶ȱȴ�������ӵ�ˮ��̶ȴ������У� c(CH3COOH)��c(Na+)���ٸ�駵���غ㣺c(CH3COO��)��c(OH��) = c(Na+)��c(H+)�����Ǿ������¹�ϵʽ��c(CH3COO��)��c(OH��) �� c(CH3COOH)��c(H+)�����ѡC��
���㣺����ˮ��͵�����й�֪ʶ��

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�25��ʱ�����и���Һ���й��������ʵ���Ũ�ȹ�ϵ��ȷ����
| A����0.1 mol��L��1Na2S��Һ�У�2c(Na��) =c(S2��)��c(HS��) ��c(H2S) |
| B��pH=2�Ĵ�����Һ��pH=12��NaOH��Һ�������ϣ� c(Na��)+ c(H��)= c(OH��)+c(CH3COO��) |
| C����0.1 mol��L��1������0.1 mol��L��1K2CO3��Һ�������ϣ� c(K��) ��c(Cl��)��c(HCO3��)��c(OH��)��c(H��) |
| D����0.1 mol��L��1NH4HSO4��Һ�еμ�NaOH����Һǡ�ó����ԣ� |
������Һ������Ũ�ȵĹ�ϵһ����ȷ����
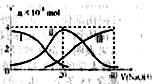
A�� �� �� ��Һ�У� ��Һ�У�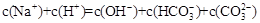 |
B��һԪ����MOH��Ӧ����MCl��Һ�У� |
C�������ʵ�����һԪ����HX�������KX�Ļ����Һ�У� |
D��pH=3��һԪ��HX��pH=11��һԪ��MOH�������ϣ� |
������Һ���й����ʵ�Ũ�ȹ�ϵ��ȷ���� ( )
| A��c��NH4������ȵ�NH4HCO3��NH4HSO4��NH4Cl��Һ�У�c (NH4HSO4) ��c(NH4HCO3) ��c(NH4Cl) |
| B�����������Һ�м����������ᣬ�õ������Ի����Һ�У�C(Na+)��C(CH3COO-)��C(H+)��C(OH-) |
| C��1.0mol/LNa2CO3��Һ�У�C(OH-)=C(HCO3-)+C (H+)+2C(H2CO3) |
| D��ij��Ԫ�������ʽ��NaHA��Һ�У�C(H+)+C(Na+)=C(OH-)+C(HA-)+C(A2-) |
�����Ƕ�Ԫ��ǿ�ᣬ����������Һ�����ԡ������£���10 mL 0.01 mol/LNaHC2O4��Һ�еμ�0.01 mol/L NaOH��Һ������NaOH��Һ��������ӣ���Һ������Ũ�ȹ�ϵ��ȷ����
| A��V��NaOH��=0ʱ��c��H+��=1��10-2mol/L |
| B��V��NaOH����10mLʱ�������ܴ���c��Na+��=2c��C2O42-��+c��HC2O4-�� |
| C��V��NaOH��=10mLʱ��c��H+��=1��10-7mol/L |
| D��V��NaOH����10mLʱ��c��Na+����c��C2O42-����c��HC2O4-�� |
�����£�������������Һ��������ȷ����
| | �� | �� | �� | �� |
| pH | 10 | 10 | 4 | 4 |
| ��Һ | ��ˮ | ����������Һ | ������Һ | ���� |
B���ڡ�������Һ��ȣ����ߵ�kw��ͬ
C���١��ڡ����зֱ���������Ĵ���粒����������Һ��pH����С
D���١�������Һ��һ������Ȼ�ϣ�������Һ������Ũ��˳��һ��Ϊ�� c(NH4+)��c(Cl��)��c(H+)�� c(OH��)
25��ʱ���й�����ĵ���ƽ�ⳣ�����£������й�˵����ȷ���� �� ��
| ���ữѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | 1.8��10��5 | 4.9��10��10 | K1=4.3��10��7 K2=5.6��10��11 |
B��a mol��L��1HCN��Һ��b mol��L��1 NaOH��Һ�������Ϻ�������Һ��c(Na+)��c(CN��)����aһ��С��b
C����0.1 mol��L��1CH3COOH��Һ����μ���ˮ������Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С
D��NaHCO3��Na2CO3�����Һ�У�һ������ c��Na+��+c��H+����c��OH����+c��HCO3����+2c��CO32����
���д�ʩ����������
| A����SO2Ư��ֽ���Ͳ�ñ�� |
| B����������ϴ��¯�е�ˮ�� |
| C���������ý�̿��ԭSiO2��ȡ�ֹ� |
| D���� Na2S������������ȥ��ˮ�е�Cu2+��Hg2+ |