��Ŀ����
25��ʱ���й�����ĵ���ƽ�ⳣ�����£������й�˵����ȷ���� �� ��
| ���ữѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | 1.8��10��5 | 4.9��10��10 | K1=4.3��10��7 K2=5.6��10��11 |
B��a mol��L��1HCN��Һ��b mol��L��1 NaOH��Һ�������Ϻ�������Һ��c(Na+)��c(CN��)����aһ��С��b
C����0.1 mol��L��1CH3COOH��Һ����μ���ˮ������Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С
D��NaHCO3��Na2CO3�����Һ�У�һ������ c��Na+��+c��H+����c��OH����+c��HCO3����+2c��CO32����
D
�������������A�����ݵ���ƽ�ⳣ����֪����ǿ��˳��Ϊ��CH3COOH��H2CO3��HCN��HCO3-������Խ������Ӧ�������������ˮ��̶�Խ����Һ��pHԽ�����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��Na2CO3����pH��NaCN����pH��NaHCO3����pH��CH3COONa������A����B����c��Na+����c��CN-����������Һ�����Կ�֪c��H+����c��OH-������Һ�ʼ��ԣ�a��b��a�Tb�����ϣ���B����C������������������ʣ���ˮ�ٽ����룬����������μ�ˮ����Һ�ĵ�������������С��pH�ȼ�С��������ĵ���̶�������C����D��Na2CO3��NaHCO3�����ʵ��������Һ�У����ڵ���������Na+��H+����������OH-��HCO3-��CO32-��������Һ�����ԣ�����C��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-������D��ȷ����ѡD��
���㣺����������ʵĵ���ƽ�⣬�漰����Ũ�ȴ�С�Ƚϡ�����ˮ���

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д������£�Ũ�Ⱦ�Ϊ0.1mol/L����Һ���ٰ�ˮ�������ᡢ���Ȼ����Һ������˵������ȷ����
| A��c(NH4+)���ۣ��� |
| B��ˮ�������c(H+)���ڣ��� |
| C���ٺ͢ڵ������Ϻ����Һ��c(NH4+)+c(NH3��H2O)��0.05mol/L |
| D���ٺ͢۵������Ϻ����ҺpH��7��c(NH4+)��c(Cl��)��c(OH��)��c(H+) |
������Һ�е�����Ũ�ȹ�ϵ��ȷ����
| A��0.1 mol/L NaHCO3��Һ�У�c(Na+)��c(HCO3-)��c(CO32-)��c(H2CO3) |
| B��1L0.1 mol/L Na2S��Һ�У�c(OH-)-c(H+)��c(HS-)+c(H2S) |
| C�������£�pH��3.5�ĸ���֭��c(H+)��pH��6.5��ţ����c(H+)��1000�� |
| D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ�У� |
����˵����ȷ����
| A���������������ܽ��С��������KSP��һ��С |
| B��ͬ�����γɵ���ʽ���ܽ��һ�������ε��ܽ�ȴ� |
| C����ΪKsp(BaSO4)= 1��08��l0��l0��Ksp(BaCO3)=8��1��10��9������BaSO4����������ת��ΪBaCO3���� |
| D����ͬ�¶��£�������AgCl����ֱ����ͬ����Ģ�0��1mo1��L��l���ᡢ��0��1mo1��L��1�Ȼ�þ��Һ����0��1mo1��L��l l��������Һ�У�c��Ag+������>��>�� |
���й��̻�����������ˮ���ص���
| A��������Һȥ���� | B��ϡ������Һ����ʱ��pH ��С |
| C��С�մ���Һ��AlCl3��Һ��ϲ�������ͳ��� | D��Ũ��������Һ�г�ζ |
����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��Һ����ˮ��Һ���������1:2��ϡ���֪Mg(OH)2��Ksp=4.0��10��12������˵����ȷ����
| A�����ǰ����ˮ��Һ��c(NH4+)��c(OH��)=1��1 |
| B����Ϻ�c(NH4+)��c(Cl��)=1��1 |
| C����Ϻ�c(Mg2+)��c(OH��)= 4.0��10��12 |
| D�����Ϻ�ķ�ɢϵ�м���FeCl3��Һ����ɫ��������ɫ |
�����£����и���������ָ����Һ��һ���ܴ����������
A�������̪��Һ�Ժ�ɫ����Һ�У�K����Na����Cu2���� |
B���������NaOH��Һ������ϡH2SO4ʱ�����ܲ�����ɫ��������Һ��Al3����Ba2����Cl����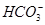 |
C��ˮ���������c(H��)��10��13mol��L��1����Һ�У�Na����Cl���� �� ��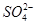 |
D��0.1 mol��L��1HNO3��Һ�У�Mg2����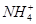 ��Fe2����Cl�� ��Fe2����Cl�� |