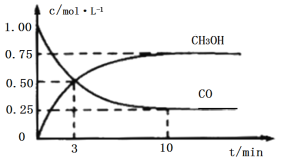
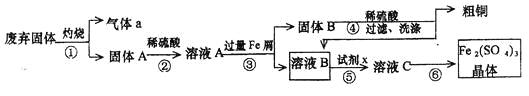
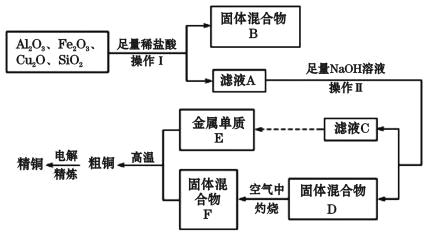
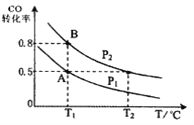
��Ŀ����
����Ŀ��ij̼�ظֹ�¯��ˮ������Ҫ�ɷ���̼��ơ�����ơ�������þ�����⡢��������ȡ�ˮ�����γɰ�ȫ�������輰ʱ��ϴ��ȥ����ϴ�������£�
����NaOH��Na2CO3���Һ�����ȣ�������Сʱ��
�ų�ϴ�ӷ�Һ����ˮ��ϴ��¯������ϡ���������NaF��Һ�����ݣ�
����ϴҺ�м���Na2SO3��Һ��
������ϴ��꣬��NaNO2��Һ�ۻ���¯��
��1����NaOH�ܽ��������Ļ�ѧ����ʽ��__________________________________��
��2����֪��20��ʱ�ܽ��/g
CaCO3 | CaSO4 | Mg(OH)2 | MgCO3 |
1.4��10-3 | 2.55��10-2 | 9��10-4 | 1.1��10-2 |
�������ݣ���ϻ�ѧƽ��ԭ��������ϴCaSO4�Ĺ���________________________��
��3���ڲ�����У�
�� ��������ˮ���������⣬����_______________________________________��
�� ��ϴ�����У��ܽ���������ٹ�¯��ʴ�������ӷ���ʽ������ԭ��________��
��4��������У�����Na2SO3��Ŀ����_______________________��
��5��������У��ۻ���Ĺ�¯����Ḳ��һ�����ܵ�Fe2O3����Ĥ��
�� ��ɲ���ƽ�䷴Ӧ�����ӷ���ʽ��______
![]()
��������ۻ�Ч���ķ�����������______��
a����¯���ϵμ�ŨH2SO4���۲���Һ�����ػ�ɫ��ʱ��
b����¯���ϵμ�����CuSO4��Һ���۲���ɫ��ʧ��ʱ��
c����¯���ϵμ�����K3[Fe(CN)6]��Һ���۲������ɫ������ʱ��
d����¯���ϵμ�ŨHNO3���۲���ֺ���ɫ�����ʱ��
���𰸡�SiO2 +2NaOH��Na2SiO3+H2O CaSO4��ˮ�д���ƽ��CaSO4��s)![]() Ca2+��aq)+ SO42-��aq)����Na2CO3��Һ���ݺ�Ca2+��CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�CaSO4ת����CaCO3��Ȼ���������ȥ CaCO3��Mg��OH)2��SiO2 2Fe3++Fe��3Fe2+ ��Fe3+��ԭ��Fe2+����ֹFe3+��ʴ��¯ 2 2 1 1 1Fe2O3 2OH- b c
Ca2+��aq)+ SO42-��aq)����Na2CO3��Һ���ݺ�Ca2+��CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�CaSO4ת����CaCO3��Ȼ���������ȥ CaCO3��Mg��OH)2��SiO2 2Fe3++Fe��3Fe2+ ��Fe3+��ԭ��Fe2+����ֹFe3+��ʴ��¯ 2 2 1 1 1Fe2O3 2OH- b c
��������
��1�����������������������NaOH��Ӧ�Ļ�ѧ����ʽ��SiO2 2NaOH��Na2SiO3 + H2O��
��2��CaSO4��ˮ�д���ƽ��CaSO4��s)![]() Ca2+��aq)+ SO42-��aq)����Na2CO3��Һ���ݺ��ݱ������ݿ��ж�Ca2+��CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�CaSO4ת����CaCO3��������������ȥ̼��Ƽ��ɡ�
Ca2+��aq)+ SO42-��aq)����Na2CO3��Һ���ݺ��ݱ������ݿ��ж�Ca2+��CO32-��ϳɸ����ܵ�CaCO3��ʹ����ƽ�����ƣ�CaSO4ת����CaCO3��������������ȥ̼��Ƽ��ɡ�
��3���ٷ����������ᷴӦ����HF��HF����������跴Ӧ�����Ա�������ˮ���������⣬����CaCO3��Mg��OH)2��SiO2��
����ϴ�����У��ܽ���������ٹ�¯��ʴ���������������Ӿ��������ԣ���������Ӧ����Ӧ�����ӷ���ʽΪ2Fe3+ + Fe��3Fe2+��
��4�������������ܸ�ʴ���������������ƾ��л�ԭ�ԣ����Բ�����У�����Na2SO3��Ŀ���ǽ�Fe3+��ԭ��Fe2+����ֹFe3+��ʴ��¯��
��5���ٸ��ݷ���ʽ��֪��Ԫ�صĻ��ϼ۴�0�����ߵ�+3�ۣ�ʧȥ3�����ӡ���Ԫ�صĻ��ϼ۴�+3�۽��͵�0�ۣ��õ�3�����ӣ����Ը��ݵ��ӵ�ʧ�غ��֪��Ӧ�ķ���ʽΪ2Fe+2NO2- +H2O��N2��+ Fe2O3+ 2OH-��
��a. ��¯���ϵμ�ŨH2SO4��Ũ������ʹ���ۻ������Բ���ͨ���۲���Һ�����ػ�ɫ��ʱ����ۻ�Ч����a����b. ����ͭ�����������û���Ӧ��������¯���ϵμ�����CuSO4��Һ��ͨ���۲���ɫ��ʧ��ʱ����Լ��ۻ�Ч����b��ȷ��c. ��������������Ӧ�����������ӣ���������������K3[Fe��CN)6]��Ӧ������ɫ���������ͨ���۲������ɫ������ʱ����Լ��ۻ�Ч����c��ȷ��d. ��¯���ϵμ�ŨHNO3��Ũ���������ۻ������Բ���ͨ���۲���ֺ���ɫ�����ʱ����ۻ�Ч����d����ѡbc��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��������X������(��ͨ��)Y��Һ�����ɳ������������Y�����ʵ�����ϵ��ͼ��ʾ���±��з���ͼʾ�������

A | B | C | D | |
X | CO2 | HCl | NaOH | AlCl3 |
Y | Ca(OH)2 | NaAlO2 | AlCl3 | NaOH |
A.AB.BC.CD.D