��Ŀ����
����Ŀ����������(ClO2)��һ�ֹ��ס���Ч����������������ˮ��������ˮ�����ȷ����й㷺Ӧ�á�������ijУ��ѧ�о�С�����ʵ����ȡClO2�Ĺ�������ͼ�������й�˵����ȷ����(����)

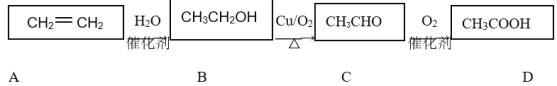
A.���ʱ������Ӧ�Ļ�ѧ����ʽΪ2HCl![]() Cl2����H2��
Cl2����H2��
B.��NaClO2��Һ��ȡ0.6 mol ClO2ʱ����������0.1 mol NCl3
C.�������Ļ������ͨ��ʢ�м�ʯ�ҵĸ������Գ�ȥClO2�е� NH3
D.��ҺX�ijɷ�ΪNaClO3��NaOH
���𰸡�B
��������
��������ͼ��֪�����ʱ�ķ�Ӧ��ΪNH4Cl��HCl������ΪH2��NCl3�����ݲ����֪��NaClO2��NCl3����������ԭ��Ӧ��ClO2Ϊ�������NH3Ϊ��ԭ�������Ԫ���غ��֪�������ﻹ��NaCl��NaOH�������Ϸ������
A.��������ͼ��֪�����ʱ�ķ�Ӧ��ΪNH4Cl��HCl������ΪH2��NCl3���������ȷ�Ļ�ѧ����ʽΪNH4Cl��2HCl![]() NCl3��3H2����A����
NCl3��3H2����A����
B.NCl3��NaClO2�������ʵ���֮��1:6ǡ�÷�Ӧ����ClO2��NH3��NaCl��NaOH����ϵ����غ��֪��ClԪ�صĻ��ϼ����ߣ���Ԫ�صĻ��ϼ۽��ͣ����ɰ���������ʽΪ��NCl3+6NaClO2+3H2O=6ClO2+3NaCl+3NaOH+NH3����NaClO2��Һ��ȡ0.6 mol ClO2ʱ����������0.1 mol NCl3����B��ȷ��
C.����Ϊ�������壬��ʯ�Ҳ������հ���������ȥNH3��������Ũ�����ȥ��������C����
D.����������ԭ��Ӧ���ɺ�Ԫ���غ��֪����ҺX����Ҫ�ɷ�ΪNaCl��NaOH����D����
�ʴ�ѡB��

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�����Ŀ��ij̼�ظֹ�¯��ˮ������Ҫ�ɷ���̼��ơ�����ơ�������þ�����⡢��������ȡ�ˮ�����γɰ�ȫ�������輰ʱ��ϴ��ȥ����ϴ�������£�
����NaOH��Na2CO3���Һ�����ȣ�������Сʱ��
�ų�ϴ�ӷ�Һ����ˮ��ϴ��¯������ϡ���������NaF��Һ�����ݣ�
����ϴҺ�м���Na2SO3��Һ��
������ϴ��꣬��NaNO2��Һ�ۻ���¯��
��1����NaOH�ܽ��������Ļ�ѧ����ʽ��__________________________________��
��2����֪��20��ʱ�ܽ��/g
CaCO3 | CaSO4 | Mg(OH)2 | MgCO3 |
1.4��10-3 | 2.55��10-2 | 9��10-4 | 1.1��10-2 |
�������ݣ���ϻ�ѧƽ��ԭ��������ϴCaSO4�Ĺ���________________________��
��3���ڲ�����У�
�� ��������ˮ���������⣬����_______________________________________��
�� ��ϴ�����У��ܽ���������ٹ�¯��ʴ�������ӷ���ʽ������ԭ��________��
��4��������У�����Na2SO3��Ŀ����_______________________��
��5��������У��ۻ���Ĺ�¯����Ḳ��һ�����ܵ�Fe2O3����Ĥ��
�� ��ɲ���ƽ�䷴Ӧ�����ӷ���ʽ��______
![]()
��������ۻ�Ч���ķ�����������______��
a����¯���ϵμ�ŨH2SO4���۲���Һ�����ػ�ɫ��ʱ��
b����¯���ϵμ�����CuSO4��Һ���۲���ɫ��ʧ��ʱ��
c����¯���ϵμ�����K3[Fe(CN)6]��Һ���۲������ɫ������ʱ��
d����¯���ϵμ�ŨHNO3���۲���ֺ���ɫ�����ʱ��