��Ŀ����
a��b��c��d��e��f��g��hΪ8���ɶ�����Ԫ�ع��ɵ��������Ƕ���10�����ӣ���ṹ�ص����£�
|
������ |
a |
b |
c |
d |
e |
f |
g |
h |
|
ԭ�Ӻ˸��� |
���� |
���� |
˫�� |
��� |
���� |
��� |
��� |
��� |
|
�����������λ����ɣ� |
0 |
+1 |
-1 |
0 |
+2 |
+1 |
0 |
+1 |
����b�����Ӱ뾶����e�����Ӱ뾶��d���ɼ��Լ����ɵ���ԭ�ӷ��ӣ�c��f���γ�����������g���ӣ�d����h����һ�������¿ɻ���ת������д����?
(1)a��ԭ�Ӻ���ṹʾ��ͼ��___________����Ԫ���������ڱ��е�_____����
c������ʽΪ ___________��?
(2)b��e��ӦԪ�ص�����������Ӧˮ����ļ���ǿ���Ƚ�Ϊ:
________��___________��д��ѧʽ��?
(3)c����f����Ӧ����2����g�����ӷ���ʽΪ_____________________��?
(4)����c���ʹ���h����������d�������ӷ�Ӧ����ʽΪ______________��?
(5)��g����ԭ���ӻ���ʽΪ_______ ��d�����ӽṹ��__________�ͣ�
��16�֣���1��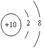 ��p ��
��p ��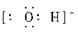 (2)
NaOH��Mg(OH)2?
(2)
NaOH��Mg(OH)2?
(3) H3O+�� OH-��2H2O?(4)NH4+��OH-��NH3����H2O?(5) sp3����������
��������
���������(1)a�ǵ���10������������a��Ne����ԭ�Ӻ���ṹʾ��ͼ�� ���������������ڰ��չ���ԭ�����������ӵĹ�����ƣ����Ը�Ԫ���������ڱ��е�p����C��˫�˴�1������ɣ����c��OH���������ʽΪ
���������������ڰ��չ���ԭ�����������ӵĹ�����ƣ����Ը�Ԫ���������ڱ��е�p����C��˫�˴�1������ɣ����c��OH���������ʽΪ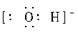 ��
��
(2)b��e�ֱ��ǵ��˴�1������ɺ�2������ɣ���ֱ��Ǻ������Ӻ�þ���Ӽ����ƵĽ�����ǿ��þ�Ľ����ԣ�������ӦԪ�ص�����������Ӧˮ����ļ���ǿ���Ƚ�ΪNaOH��Mg(OH)2��
(3)f�Ƕ�˴�1������ɣ���c����f����Ӧ����2����g����������f��H3O+���÷�Ӧ�����ӷ���ʽΪH3O+�� OH-��2H2O��
(4) h�Ƕ�˴�1������ɣ���h��NH4+�����Դ���c���ʹ���h����������d�������ӷ�Ӧ����ʽΪNH4+��OH-��NH3����H2O��
(5)��g��ˮ���ռ乹����V�νṹ����������ԭ���ӻ���ʽΪsp3���������ӽṹ�������͡�
���㣺����10����������ѧʽ������ʽ��ԭ�ӽṹʾ��ͼ������ʽ���ռ乹���Լ��ӻ�������͵��й��ж�
�������������е��Ѷȵ����⣬���������ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������Ĺؼ���������ס������10��������Ȼ��������������ü��ɣ�����������ѧ�����������ͳ���˼ά���������ѧ����Ӧ��������ѧϰЧ�ʡ�

 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�