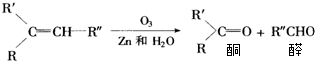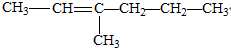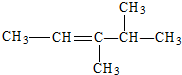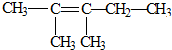��Ŀ����
1��A��B��C��D��E���������к���ͬһ��Ԫ�أ����ת����ϵ����ͼ��ʾ������A��B��C��D�ڳ����¶������壬BΪ����ɫ��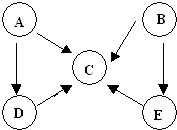
��1������ȷ�Ļ�ѧ����������B�ķ���ʽ��NO2��A�ĵ���ʽ��
 ��D�Ľṹʽ��
��D�Ľṹʽ�� ��
����2��д�����з�Ӧ�Ļ�ѧ����ʽ��
D��C�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��
E��B�����ӷ���ʽΪCu+4H++2NO3-�TCu2++2H2O+NO2��
A��D��ѧ����ʽΪN2+3H2$\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$=2NH3��
���� BΪ����ɫ�����壬��BӦΪNO2��A��B��C��D��E���������к���ͬһ��Ԫ�أ���Ӧ�ú���NԪ�أ���A��B��C��D�ڳ����¶������壬���ݿ�ͼת����ϵ��֪��AΪN2��BΪNO2��CΪNO��DΪNH3��E�������壬���������ܹ�ת����E��E�ܹ�����NO����Eֻ��ΪHNO3���ݴ˽��н��
��� �⣺BΪ����ɫ�����壬��BӦΪNO2��A��B��C��D��E���������к���ͬһ��Ԫ�أ���Ӧ�ú���NԪ�أ���A��B��C��D�ڳ����¶������壬���ݿ�ͼת����ϵ��֪��AΪN2��BΪNO2��CΪNO��DΪNH3��E�������壬���������ܹ�ת����E��E�ܹ�����NO����Eֻ��ΪHNO3��
��1�����ݷ�����֪��B�ķ���ʽΪ��NO2��AΪ�����������д��ڵ��������������ʽΪ�� ��DΪ�����������д���3��������������ĽṹʽΪ��
��DΪ�����������д���3��������������ĽṹʽΪ�� ��
��
�ʴ�Ϊ��NO2�� ��
�� ��
��
��2��D��CΪ��������������NO��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��
E��BΪ����ת���ɶ����������÷�Ӧ����Ϊͭ��Ũ���ᷴӦ���÷�Ӧ�����ӷ���ʽ����Ϊ��Cu+4H++2NO3-�TCu2++2H2O+NO2����
A��DΪ�����ڸ��¡���ѹ������������ת���ɰ������÷�Ӧ�Ļ�ѧ����ʽΪ��N2+3H2$\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$=2NH3��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��Cu+4H++2NO3-�TCu2++2H2O+NO2����N2+3H2$\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$=2NH3��
���� ���⿼�����ƶϣ���Ŀ�Ѷ��еȣ�����Ĺؼ���������Ļ������������ͻ�ƿڣ��������������ɣ��������ó���ȷ�Ĵ𰸣��籾���к���ɫ������BΪͻ�ƿڣ�ע�����ճ���Ԫ�ص��ʼ��仯��������ʣ�

| A�� | AlCl3��Һ�л����ܴ������ڣ�H+��NH4+��SO42-��NO3- | |
| B�� | AlCl3��Һ������İ�ˮ��Ӧ�����ӷ���ʽΪ��Al3++4NH3•H2O�T4NH4++AlO2-+2H2O | |
| C�� | ��NAΪ����٤��������ֵ����1L0.1mol•L-1��AlCl3��Һ��Al3+����ĿΪ0.1NA | |
| D�� | ��ҵ�Ͽ��õ�����ڵ�AlCl3����ȡ����Al |
| A�� | ${\;}_{2}^{3}$He����ԭ�Ӻ�����2�����Ӻ�3�����ӵĺ�ԭ�� | |
| B�� | ${\;}_{2}^{3}$He��${\;}_{2}^{4}$He�ֱ���1��2������ | |
| C�� | ${\;}_{2}^{3}$He ��${\;}_{2}^{4}$He��Ϊͬλ�� | |
| D�� | ${\;}_{2}^{3}$He������������Ϊ1������${\;}_{2}^{3}$He ���н�ǿ�Ľ����� |
��1��CO��������������֪��Fe2O3��s��+3C��s���T2Fe��s��+3CO��g����H1=+489.0kJ•mol-1��C��s��+CO2��g���T2CO��g����H2=+172.5kJ•mol-1����CO��ԭFe2O3��s�����Ȼ�ѧ����ʽΪFe2O3��s��+3CO��g��=2Fe��s��+3CO2��g����H=-28.5kJ•mol-1��
��2��CO��O2��Ƴ�ȼ�ϵ�أ���KOH��ҺΪ���Һ�����õ�صĸ�����ӦʽΪCO+4OH--2e-=CO32-+2H2O��
��3��CO2��H2����һ������ĺ����ܱ������У��������¶��·�����Ӧ��
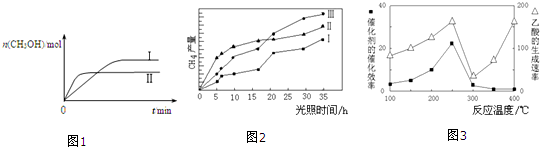
CO2��g��+3H2��g��?CH3OH��g��+H2O��g�������CH3OH�����ʵ�����ʱ��ı仯��ͼ1��
�ٸ÷�Ӧ�ġ�HС��0������ڻ�С�ڡ���������I�����Ӧ��ƽ�ⳣ����С��ϵΪK����K���������=������
��һ���¶��£����ݻ���ͬ�ҹ̶��������ܱ������У������·�ʽ���뷴Ӧ�һ��ʱ���ﵽƽ�⣮
| �ݡ����� | �� | �� |
| ��Ӧ��Ͷ���� | 1mol CO2��3mol H2 | a��mol CO2��3a��mol H2�� b��mol CH3OH��g����b��mol H2O��g�� |
��4����TiO2/Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᣮ�ڲ�ͬ�¶��´����Ĵ�Ч����������������ʵĹ�ϵ��ͼ3��
�ٵ��¶���300�桫400�淶Χʱ���¶��������������ʵ���ҪӰ�����أ�
��Cu2Al2O4������ϡ���ᣬϡ���ỹԭ����ΪNO��д���йص����ӷ���ʽ3Cu2Al2O4+32H++2NO3-=6Cu2++6Al3++2NO��+16H2O��
��Ũ�����е���ʯ����Һ����
��ͭ��Ũ���ᷴӦ
������������Ũ���ᷴӦ
��̼��Ũ���ᣮ
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ڢ� |
| A�� | CCl4 | B�� | CH3COOH | C�� | CH3CH2OH | D�� | CaCO3 |
| A�� | CO32- | B�� | SO42- | C�� | Ag+ | D�� | SO32- |
| A�� | �Ȼ�����Һ����ˮ | B�� | NaOH��Һ����ˮ | ||
| C�� | ���������Һ����ˮ | D�� | NaHCO3��Һ����ˮ |