��Ŀ����
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ���ͼ��Ԫ�����ڱ���һ���֡�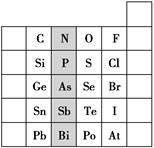
(1)��Ӱ����Ԫ����Ԫ�����ڱ��е�λ��Ϊ________�塣
(2)����Ԫ��������Ԥ�⣺H3AsO4����ǿ�ỹ�����________��
(3)C��SiԪ�ص��⻯�ﶼ����ȼ�գ���SiԪ�ص��⻯���ڿ����п�����ȼ����ԭ����_________________________________________��
��д��Si���⻯����ȫȼ�յĻ�ѧ����ʽ��______________________________��
(4)O2��H2�ķ�Ӧ�Dz����淴Ӧ����S��H2��Ӧ��һ���ȣ���д��Se��H2��Ӧ�Ļ�ѧ����ʽ��______________________
(5)�ԱȽ�S��O��F����Ԫ�ص�ԭ�Ӱ뾶��С��________(��Ԫ�ط���)��
(1)�ڢ�A�塡(2)���ᡡ(3)SiH4���ȶ���С��CH4��SiH4��2O2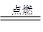 SiO2��2H2O��(4)Se��H2
SiO2��2H2O��(4)Se��H2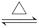 H2Se��(5)S��O��F
H2Se��(5)S��O��F
����

��ϰ��ϵ�д�
�����Ŀ
X��Y��Z��W��T��Ԫ�����ڱ���ǰ�����ڵ�����Ԫ�ء��й�������Ԫ�ص���Ϣ���±���
| Ԫ�ش��� | �����Ϣ |
| X | ����һ�ֺ��ص�ԭ�Ӻ���û������ |
| Y | ����ɺܶ������ʲ�ͬ�ĵ��ʣ�Ҳ���γɻ�������������һ��Ԫ�� |
| Z | ��̬ԭ�ӵ����������Ų�ʽΪnsnnpn+1 |
| W | ����Ԫ����������ֻ������Щ��������ˮ��Ӧ�����ɼ����ƽ������W3����Ϊ�����������ɡ�� |
| T | ��Ҫ���ϼ���+1��+2�ۣ������������ˮ��Һ�г���ɫ |
���Ƴ�����Ԫ�أ��û�ѧ����ش��������⣺
��1��д��WԪ�������ڱ��е�λ��____________��д��TԪ�صĻ�̬�����Ų�ʽ________________��
��2����������Ԫ���е縺��������________,Y Z W����Ԫ�صĵ�һ������˳��Ϊ_______________��
��3����X Z ����Ԫ�ؿ����γ�һ��ZX5�ļȺ����Ӽ��ֺ����ۼ��Ļ���������ʽΪ__________��
��4��TW�ڸ�����������һ�������������������ܽ��ܶ��л���������ȫ����(�൱����ȫȼ��)��д�����������£�TW��Y����⻯�ﷴӦ�Ļ�ѧ����ʽ��_______________________________________��
��5����֪��25�桢101 kPa�£�
XYZ(aq)+X2W��1��
 YZ- (aq)+X3W+(aq) ��H="+45.6" KJ/mol
YZ- (aq)+X3W+(aq) ��H="+45.6" KJ/molX3W+(aq)+WX(aq)=2X2W��1�� ��H="-57.3" KJ/mol
����25�桢101 kPa��ϡ��Һ�У�XYZ��WX��Ӧ���Ȼ�ѧ����ʽ(�����ӷ���ʽ��ʾ) ��_________��
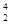 He��
He��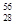 Ni��
Ni��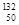 Sn��Pb����Ϊ�ȶ������ݴ���Ϣ�ش��������⣺
Sn��Pb����Ϊ�ȶ������ݴ���Ϣ�ش��������⣺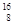 O��
O��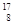 O��
O��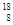 O���������������������ɣ�����ͬλ����ԭ�Ӻ����ȶ�����________��
O���������������������ɣ�����ͬλ����ԭ�Ӻ����ȶ�����________�� Rλ��Ԫ�����ڱ��ĵ�________���ڵ�________�壬���ȶ���
Rλ��Ԫ�����ڱ��ĵ�________���ڵ�________�壬���ȶ���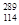 R(�����������������)��
R(�����������������)��