��Ŀ����
7������������������������ж��ַ�������NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ���������Ӧԭ����ͼ1��ʾ��
����ͼ��֪SCR�����е�������ΪNO��NO2��
����Fe������ʱ���ڰ�������������£���$\frac{c��N{O}_{2}��}{c��NO��}$=1��1ʱ���ѵ�����ѣ���֪ÿ����28g N2 �ų�������ΪQkJ���÷�Ӧ���Ȼ�ѧ����ʽΪ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H=-2QkJ/mol��
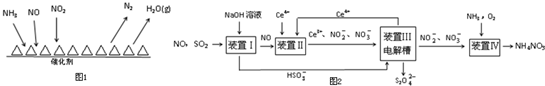
��ҵ�ϱ䡰�ϡ�Ϊ�������չ�ҵ��SO2��NO���ɻ��Na2S2O4��NH4NO3��Ʒ������ͼ��ͼ2��CeΪ��Ԫ�أ���
��װ�â��е���Ҫ��Ӧ�����ӷ���ʽΪSO2+OH-=HSO3-��
��װ�â���ʹCe4+���������ü���ȼ�ϵ�ص���װ���е���Һ��������1mol CH4ʱ�������Ͽ�����8mol Ce4+��
���û���̿��ԭ�����Դ�����������緢����Ӧ��C��s��+2NO��g��?N2��g��+CO2��g����H=Q kJ/mol��
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ�䣨min�� Ũ�ȣ�mol/L�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
| N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
| CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ͨ��һ������NO���ʵ���С���������������ѹǿ��ͨ������ʵ�����CO2��N2����һ�ּ��ɣ���
���� I���ٵõ��ӻ��ϼ۽��͵ķ�Ӧ������������
������1mol�����ų�QkJ������������2mol�����ų�2QkJ������
��װ�â��з�������������NaOH�ķ�Ӧ��
����װ�â���Ce3+-e-�TCe4+���ü���ȼ�ϵ�ص���װ���е���Һ����CH4��8e-��8Ce4+��
��Tl��ʱ���ɱ������ݿ�֪20min�ﵽƽ�⣬ƽ��Ũ��c��N2��=0.3mol/L��c��CO2��=0.3mol/L��c��NO��=0.4mol/L���Դ˼��㷴Ӧ��ƽ�ⳣ����
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬C��s��+2NO��g��?N2��g��+CO2��g������ͼ�����ݷ�����ƽ��״̬����Ũ������ƽ�ⳣ��K��T$\frac{0.36��0.36}{��{0.48��}^{2}}$=$\frac{9}{16}$��ƽ�ⳣ�����䣬����Ũ�������Ӱ�����������
��� �⣺�ٸ���ͼ��֪����Ӧ����NO��NO2��NH3����������N2��H2O������������NԪ�ػ��ϼ��������ϼ۱�Ϊ0�ۡ�������NԪ�ػ��ϼ���-3�۱�Ϊ0�ۣ�������������NO��NO2���ʴ�Ϊ��NO��NO2��
�ڵ�$\frac{c��N{O}_{2}��}{c��NO��}$=1��1ʱ���ѵ�����ѣ�ÿ����28g N2 �ų�������ΪQkJ��������1mol�����ų�QkJ������������2mol�����ų�2QkJ�����������Ȼ�ѧ��Ӧ����ʽΪ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H=-2QkJ/mol��
�ʴ�Ϊ��2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H=-2QkJ/mol��
��װ�â��з�������������NaOH�ķ�Ӧ�����ӷ�ӦΪSO2+OH-=HSO3-��NO����������֮�䲻�ᷴӦ���ʴ�Ϊ��SO2+OH-=HSO3-��
����װ�â���Ce3+-e-�TCe4+���ü���ȼ�ϵ�ص���װ���е���Һ���ɵ����غ��֪CH4��8e-��8Ce4+��������1mol CH4ʱ�������Ͽ�����8mol Ce4+���ʴ�Ϊ��8��
�� �٢�Tl��ʱ���ɱ������ݿ�֪20min�ﵽƽ�⣬ƽ��Ũ��c��N2��=0.3mol/L��c��CO2��=0.3mol/L��c��NO��=0.4mol/L����K=$\frac{0.3��0.3}{��0.4��^{2}}$=$\frac{9}{16}$��
�ʴ�Ϊ��$\frac{9}{16}$��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬C��s��+2NO��g��?N2��g��+CO2��g������ͼ�����ݷ�����ƽ��״̬����Ũ������ƽ�ⳣ��K��T$\frac{0.36��0.36}{��{0.48��}^{2}}$=$\frac{9}{16}$��ƽ�ⳣ�����¶ȱ仯��ƽ�ⳣ������˵���ı������һ�������¶ȣ��ɵ���Ũ����������̼��һ������Ũ������Ӧǰ������������䣬��ı������������ͨ��һ������NO���ʵ���С���������������ѹǿ��ͨ������ʵ�����CO2��N2��
�ʴ�Ϊ��ͨ��һ������NO���ʵ���С���������������ѹǿ��ͨ������ʵ�����CO2��N2��
���� ���⿼����ۺϣ��漰������ԭ��Ӧ����ѧƽ����㡢ԭ��صȣ����ػ�ѧ��Ӧԭ�����ۺ�Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�| A�� | �۱�ϩ�Ľṹ��ʽ��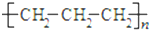 | |
| B�� | ��Ȳ�ķ��ӽṹģ��ʾ��ͼ�� | |
| C�� | H2O2�ĵ���ʽ��H+[${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$]2-H+ | |
| D�� |  �����ƣ�3-��-1-���� �����ƣ�3-��-1-���� |
| A�� | �ؽ�������Ag+��Cu2+��K+���ɵ��µ����ʱ��� | |
| B�� | S02��NxOy�����ڷǽ��������Ҳ�������������� | |
| C�� | ���ֿ�����ɢ�ڿ����п��ܻ��γɶ�������� | |
| D�� | ���ͱ��������ﶼ�Ƿ����� |
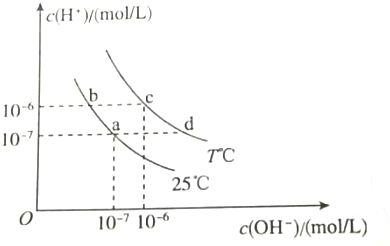
| A�� | a���Ӧ����Һ�У�Al3+��Na+��Cl-��CO32- | |
| B�� | b���Ӧ����Һ�У�K+��Ba2+��NO3-��AlO2- | |
| C�� | c���Ӧ����Һ�У�Fe3+��Na+��I-��SO42- | |
| D�� | d���Ӧ����Һ�У�Na+��K+��SO32-��Cl- |
| A�� | �����Ρ�sp3 | B�� | V�Ρ�sp2 | C�� | ƽ�������Ρ�sp2 | D�� | �����Ρ�sp2 |
| A�� | O | B�� | P | C�� | Si | D�� | Fe3+ |
 ijͬѧ�������ͼ��ʾ����ʵ��װ�ã�����װ��δ���������Ʊ�SO2������ʵ�������������仹ԭ�ԣ��Ʊ�SO2ʱѡ�õ��Լ�ΪCu��ŨH2SO4���ش��������⣺
ijͬѧ�������ͼ��ʾ����ʵ��װ�ã�����װ��δ���������Ʊ�SO2������ʵ�������������仹ԭ�ԣ��Ʊ�SO2ʱѡ�õ��Լ�ΪCu��ŨH2SO4���ش��������⣺