��Ŀ����
15���ҹ����������ֳ��г���������������Ҫ�ɷ�Ϊϸ������SO2��NxOy���ؽ������Ӽ��������ȣ��Ի���Σ������������Խ��Խ�ܵ����ӣ�����˵����ȷ���ǣ�������| A�� | �ؽ�������Ag+��Cu2+��K+���ɵ��µ����ʱ��� | |
| B�� | S02��NxOy�����ڷǽ��������Ҳ�������������� | |
| C�� | ���ֿ�����ɢ�ڿ����п��ܻ��γɶ�������� | |
| D�� | ���ͱ��������ﶼ�Ƿ����� |
���� A��K+�����ؽ������ӣ�
B��NxOy���δ֪����һ�������������
C��������ж����ЧӦ��
D��������ͨ��ָ�����к��б����ṹ��̼�⻯���
��� �⣺A��K+�����ؽ������ӣ����ɵ��µ����ʱ��ԣ���A����
B����������Ϊ�����������NxOy���δ֪����һ���������������B����
C�������ɢ������ֱ����СΪ1-100���ף�ֱ����1-100���Ŀ������ɢ�ڿ����п��γɽ��壬��C��ȷ��
D��������������ܺ���������һ����������D����
��ѡC��
���� ���⿼���˵����ʡ���������ʡ�������������������Ķ��壬�漰֪ʶ��㣬��Ϊ������֪ʶ�������������ʵ����ʺ����ǽ���Ĺؼ���

��ϰ��ϵ�д�
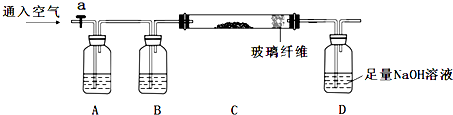
�����Ŀ
5���Ա����й����ӷ���ʽ��д���ۺ������ǣ�������
| ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
| A | Fe3O4�����ϡ���ᷴӦ��Fe3O4+8H+��2Fe3++Fe2++4H2O | ��ȷ |
| B | �ڸ���NH4Fe��SO4��2��Һ����μ���Ba��OH��2��Һ�� 2Fe3++3SO42-+3Ba2++6OH-��3BaSO4��+2Fe��OH��3�� | ��ȷ |
| C | ��ϡ��ˮ��ͨ�����CO2��NH3•H2O+CO2��NH4++HCO3- | ��ȷ |
| D | FeBr2��Һ������ʵ�����Cl2��Ӧ�� 2Fe2++2Br-+2Cl2��2Fe3++4Cl-+Br2 | ����Fe2+��Br-�Ļ�ѧ������֮��ӦΪ1��2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
10��ͭ��������Ȼͬλ�أ���${\;}_{29}^{63}$Cu+��${\;}_{29}^{65}$Cu������ͬ�ģ�������
| A�� | ������ | B�� | ������ | C�� | �˵���� | D�� | �ܲ���ܼ� |
7������������������������ж��ַ�����
��NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ���������Ӧԭ����ͼ1��ʾ��
����ͼ��֪SCR�����е�������ΪNO��NO2��
����Fe������ʱ���ڰ�������������£���$\frac{c��N{O}_{2}��}{c��NO��}$=1��1ʱ���ѵ�����ѣ���֪ÿ����28g N2 �ų�������ΪQkJ���÷�Ӧ���Ȼ�ѧ����ʽΪ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H=-2QkJ/mol��
��ҵ�ϱ䡰�ϡ�Ϊ�������չ�ҵ��SO2��NO���ɻ��Na2S2O4��NH4NO3��Ʒ������ͼ��ͼ2��CeΪ��Ԫ�أ���
��װ�â��е���Ҫ��Ӧ�����ӷ���ʽΪSO2+OH-=HSO3-��
��װ�â���ʹCe4+���������ü���ȼ�ϵ�ص���װ���е���Һ��������1mol CH4ʱ�������Ͽ�����8mol Ce4+��
���û���̿��ԭ�����Դ�����������緢����Ӧ��C��s��+2NO��g��?N2��g��+CO2��g����H=Q kJ/mol��
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��Tl��ʱ���÷�Ӧ��ƽ�ⳣ��K=$\frac{9}{16}$��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ͨ��һ������NO���ʵ���С���������������ѹǿ��ͨ������ʵ�����CO2��N2����һ�ּ��ɣ���
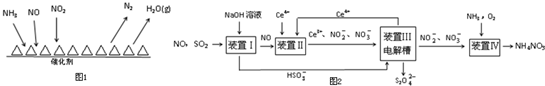
��NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ���������Ӧԭ����ͼ1��ʾ��
����ͼ��֪SCR�����е�������ΪNO��NO2��
����Fe������ʱ���ڰ�������������£���$\frac{c��N{O}_{2}��}{c��NO��}$=1��1ʱ���ѵ�����ѣ���֪ÿ����28g N2 �ų�������ΪQkJ���÷�Ӧ���Ȼ�ѧ����ʽΪ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H=-2QkJ/mol��
��ҵ�ϱ䡰�ϡ�Ϊ�������չ�ҵ��SO2��NO���ɻ��Na2S2O4��NH4NO3��Ʒ������ͼ��ͼ2��CeΪ��Ԫ�أ���
��װ�â��е���Ҫ��Ӧ�����ӷ���ʽΪSO2+OH-=HSO3-��
��װ�â���ʹCe4+���������ü���ȼ�ϵ�ص���װ���е���Һ��������1mol CH4ʱ�������Ͽ�����8mol Ce4+��
���û���̿��ԭ�����Դ�����������緢����Ӧ��C��s��+2NO��g��?N2��g��+CO2��g����H=Q kJ/mol��
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ�䣨min�� Ũ�ȣ�mol/L�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
| N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
| CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ͨ��һ������NO���ʵ���С���������������ѹǿ��ͨ������ʵ�����CO2��N2����һ�ּ��ɣ���

11������ʵ���������ʼ�ֱ��ת����Ԫ���ǣ����������ʡ������������������
| A�� | ̼ | B�� | �� | C�� | �� | D�� | �� |
 ��
��