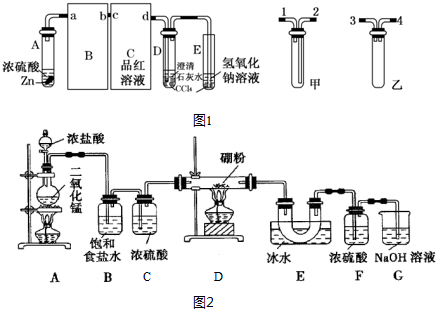
��Ŀ����
2�� ijͬѧ�������ͼ��ʾ����ʵ��װ�ã�����װ��δ���������Ʊ�SO2������ʵ�������������仹ԭ�ԣ��Ʊ�SO2ʱѡ�õ��Լ�ΪCu��ŨH2SO4���ش��������⣺
ijͬѧ�������ͼ��ʾ����ʵ��װ�ã�����װ��δ���������Ʊ�SO2������ʵ�������������仹ԭ�ԣ��Ʊ�SO2ʱѡ�õ��Լ�ΪCu��ŨH2SO4���ش��������⣺��1��д����ȡSO2�Ļ�ѧ����ʽ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CuSO4+SO2��+2H2O��
��2���÷�Ӧ��ŨH2SO4���ֵĻ�ѧ�����������ԡ����ԣ�
��3����ͬѧ����ʵ������������SO2�Ļ�ԭ�ԣ���ѡ�õ��Լ�ΪC������ѡ�������SO2��ԭ�Ե��Թ��е�����Ϊ���Ը��������Һ��ɫ��
A��˫��ˮ��H2O2��B��Ʒ����Һ C�����Ը��������Һ��
���� ��1��Cu��Ũ�����ڼ��������·�Ӧ��������ͭ������������ˮ��
��2����Ӧ��������SԪ�ز��ֻ��ϼ۽������ɶ���������δ�仯��������ͭ��
��3������ʵ���������SO2�Ļ�ԭ�ԣ�Ӧѡ���о���ǿ�����Ե��Լ����ҷ�Ӧ�������ԣ�
��� �⣺��1��Cu��Ũ�����ڼ��������·�Ӧ��������ͭ������������ˮ����Ӧ����ʽΪ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CuSO4+SO2��+2H2O��
��2����Ӧ��������SԪ�ز��ֻ��ϼ۽������ɶ���������δ�仯��������ͭ����Ӧ��������֣������ԡ����ԣ�
�ʴ�Ϊ�������ԡ����ԣ�
��3������ʵ���������SO2�Ļ�ԭ�ԣ�˫��ˮ�����Ը��������Һ����ǿ�����ԣ�����������˫��ˮ��Ӧû����������ѡ�����Ը��������Һ�������ǣ����Ը��������Һ��ɫ��
�ʴ�Ϊ��C�� ���Ը��������Һ��ɫ��
���� ���⿼��Ũ����������Ʊ�������ʵ�飬�ѶȲ����ؿ���ѧ����֪ʶ��Ǩ��Ӧ�ã������ڻ���֪ʶ�Ĺ��̣�

��ϰ��ϵ�д�
�����Ŀ
7������������������������ж��ַ�����
��NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ���������Ӧԭ����ͼ1��ʾ��
����ͼ��֪SCR�����е�������ΪNO��NO2��
����Fe������ʱ���ڰ�������������£���$\frac{c��N{O}_{2}��}{c��NO��}$=1��1ʱ���ѵ�����ѣ���֪ÿ����28g N2 �ų�������ΪQkJ���÷�Ӧ���Ȼ�ѧ����ʽΪ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H=-2QkJ/mol��
��ҵ�ϱ䡰�ϡ�Ϊ�������չ�ҵ��SO2��NO���ɻ��Na2S2O4��NH4NO3��Ʒ������ͼ��ͼ2��CeΪ��Ԫ�أ���
��װ�â��е���Ҫ��Ӧ�����ӷ���ʽΪSO2+OH-=HSO3-��
��װ�â���ʹCe4+���������ü���ȼ�ϵ�ص���װ���е���Һ��������1mol CH4ʱ�������Ͽ�����8mol Ce4+��
���û���̿��ԭ�����Դ�����������緢����Ӧ��C��s��+2NO��g��?N2��g��+CO2��g����H=Q kJ/mol��
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��Tl��ʱ���÷�Ӧ��ƽ�ⳣ��K=$\frac{9}{16}$��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ͨ��һ������NO���ʵ���С���������������ѹǿ��ͨ������ʵ�����CO2��N2����һ�ּ��ɣ���
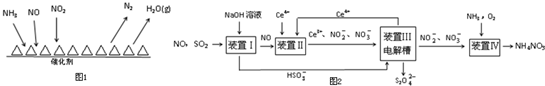
��NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ���������Ӧԭ����ͼ1��ʾ��
����ͼ��֪SCR�����е�������ΪNO��NO2��
����Fe������ʱ���ڰ�������������£���$\frac{c��N{O}_{2}��}{c��NO��}$=1��1ʱ���ѵ�����ѣ���֪ÿ����28g N2 �ų�������ΪQkJ���÷�Ӧ���Ȼ�ѧ����ʽΪ2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H=-2QkJ/mol��
��ҵ�ϱ䡰�ϡ�Ϊ�������չ�ҵ��SO2��NO���ɻ��Na2S2O4��NH4NO3��Ʒ������ͼ��ͼ2��CeΪ��Ԫ�أ���
��װ�â��е���Ҫ��Ӧ�����ӷ���ʽΪSO2+OH-=HSO3-��
��װ�â���ʹCe4+���������ü���ȼ�ϵ�ص���װ���е���Һ��������1mol CH4ʱ�������Ͽ�����8mol Ce4+��
���û���̿��ԭ�����Դ�����������緢����Ӧ��C��s��+2NO��g��?N2��g��+CO2��g����H=Q kJ/mol��
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ�䣨min�� Ũ�ȣ�mol/L�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
| N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
| CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ͨ��һ������NO���ʵ���С���������������ѹǿ��ͨ������ʵ�����CO2��N2����һ�ּ��ɣ���

11��������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | pH��ȵĢ�NH4Cl�ڣ�NH4��2SO4��NH4HSO4��Һ��c��NH4+����С˳��Ϊ�٣��ڣ��� | |
| B�� | 0.2mol•L-1CH3COOH��Һ��0.2mol•L-1CH3COONa��Һ�������ϣ�c��CH3COOH��+c��CH3COO-��=2c��Na+�� | |
| C�� | 0.1mol•L-1NaHCO3��Һ�У�c��Na+��+c��H+��=c��HCO3-��+c��CO32-��+c��OH-�� | |
| D�� | 0.1mol•L-1NaHA��Һ����pH=4��c��HA-����c��H+����c��H2A����c��A2-�� |
14���������ƣ�CaO2����һ�ְ�ɫ�ᾧ���ĩ��������ˮ�������ڴ��ࡢ���ѵȣ�������350�����ҿ�ʼ�ֽ�ų���������ˮ������Ӧ����H2O2�������ᷴӦ����H2O2���������ƿ����ڸ���ˮ�ʡ��������ؽ������ӷ�ˮ�������ೱ��Ҳ������Ӧ�������ȣ���һ����Ҫ�����Լ���
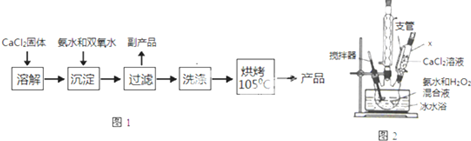
����CaO2���Ʊ�ԭ����CaCl2+H2O2+2NH3•H2O+6H2O�TCaO2•8H2O��+2NH4Clʵ�鲽����ͼ1����Ӧװ����ͼ2��ʾ����ش��������⣺
��1������x������Ϊ��ѹ��Һ©�������ѹ��Һ©����
��2����ƽ���ƶ�ԭ�����ͼ��백ˮ���������кͷ�Ӧ���ɵ�HCl��ʹCaCl2+H2O2?CaO2+2HCl���ҽ��У�
��3��������Ӧʱ���ñ�ˮԡ�����¶���0�����ң������ԭ���Ǽ���˫��ˮ���ȷֽ⡢���Ͳ����ܽ�ȱ���������д�����֣�
��4�����˺�ϴ�ӳ������Լ������B
A����ˮ����B����ˮ������C���Ҵ�������D������
����CaO2���ȼ�⣬��һ����CaO2����ϡ���ᣬ�ñ�KMnO4���ڵζ����ɵ�H2O2��KMnO4��Ӧ������Mn2+������ȷ��CaO2�ĺ�����
��5����ÿ�γ�ȡ0.4000g��Ʒ�ܽ����0.1000mol/L��KMnO4��Һ�ζ��������������ʾ����CaO2��Ʒ�Ĵ���90.00%
��6�����CaO2����ƫ�͵�ԭ�������AD
A���濾ʱ�䲻��
B���ڽྻ�������ʽ�ζ�����δ��ϴ��װ��Һ
C���ζ�ǰ���촦�����ݣ��ζ�����ʧ
D������KMnO4����Һ����ʱ��������ƿ���ߣ�

����CaO2���Ʊ�ԭ����CaCl2+H2O2+2NH3•H2O+6H2O�TCaO2•8H2O��+2NH4Clʵ�鲽����ͼ1����Ӧװ����ͼ2��ʾ����ش��������⣺
��1������x������Ϊ��ѹ��Һ©�������ѹ��Һ©����
��2����ƽ���ƶ�ԭ�����ͼ��백ˮ���������кͷ�Ӧ���ɵ�HCl��ʹCaCl2+H2O2?CaO2+2HCl���ҽ��У�
��3��������Ӧʱ���ñ�ˮԡ�����¶���0�����ң������ԭ���Ǽ���˫��ˮ���ȷֽ⡢���Ͳ����ܽ�ȱ���������д�����֣�
��4�����˺�ϴ�ӳ������Լ������B
A����ˮ����B����ˮ������C���Ҵ�������D������
����CaO2���ȼ�⣬��һ����CaO2����ϡ���ᣬ�ñ�KMnO4���ڵζ����ɵ�H2O2��KMnO4��Ӧ������Mn2+������ȷ��CaO2�ĺ�����
��5����ÿ�γ�ȡ0.4000g��Ʒ�ܽ����0.1000mol/L��KMnO4��Һ�ζ��������������ʾ����CaO2��Ʒ�Ĵ���90.00%
| ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
| ����KMnO4���/mL | 19.98 | 20.02 | 20.20 | 20.00 |
A���濾ʱ�䲻��
B���ڽྻ�������ʽ�ζ�����δ��ϴ��װ��Һ
C���ζ�ǰ���촦�����ݣ��ζ�����ʧ
D������KMnO4����Һ����ʱ��������ƿ���ߣ�
11������ʵ���������ʼ�ֱ��ת����Ԫ���ǣ����������ʡ������������������
| A�� | ̼ | B�� | �� | C�� | �� | D�� | �� |
12������˵����ȷ���ǣ�������
| A�� | ��������Ԫ�ص���������ϼ۶����������������� | |
| B�� | ���й���Ԫ�ض��ǽ���Ԫ�أ����еĽ���Ԫ��Ҳ���ǹ���Ԫ�� | |
| C�� | �����ڱ�������Ԫ�صĵ���ȫ�������� | |
| D�� | ͬ��������Ԫ�ص�ԭ�Ӱ뾶�Ԣ�A���Ϊ��С |
 ��
��