��Ŀ����
��������ȡ�봢��������Դ����������о��ȵ㡣
��1����֪��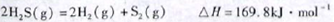
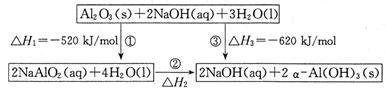
�����йظ÷�Ӧ��������ȷ����
| A������Ӧ���С��169.8kJ��mol-1 |
| B���淴Ӧ���һ��С��169.8kJ��mol-1 |
| C������Ӧ��ܲ�С��169.8kJ��mol-1 |
| D������Ӧ��ܱ��淴Ӧ���С169.8kJ��mol-1 |
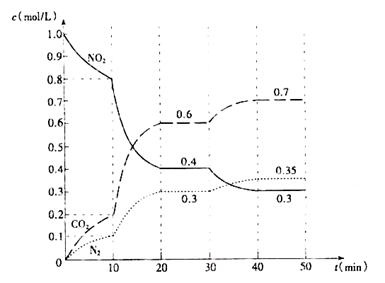
��3��H2O���ȷֽ�Ҳ�ɵõ�H2��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ����ͼ1��ʾ����4000��~5000��ʱ���ܷ���������Щ��Ӧ ����д��ĸ����
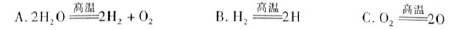
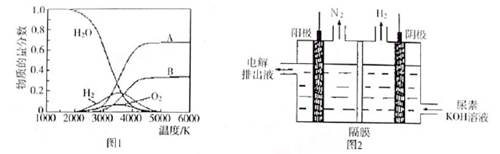
��4����ȡ��������һ�ַ����ǵ������[CO��NH2��2]�ļ�����Һ��װ��ʾ��ͼ��ͼ2�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�����õ��װ���е���ų�Һ�е���Ҫ�ɷ��� ��д��ѧʽ����
��5����֪�������ʵ�KSP��
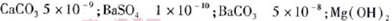
5.6��10-12��Ca��OH��2��1.4��10-5���ȼҵ�� ��ⱥ��ʳ��ˮҲ�ܵõ�������������õ���ˮ�辫�ƣ�ȥ����Ӱ���Ca2+��Mg2+��NH4+��SO42��[c��SO42����>c(Ca2+)]��ij�����������£�
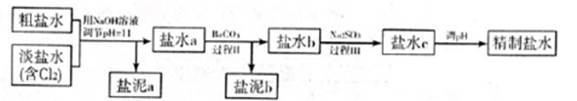
������a����ɳ�⣬�����е������� ��
�ڹ���I�н�NH4+ת��ΪN2�����ӷ���ʽ�� ��
�۹���II�г�ȥ�������� ��
�ܾ�����III������������ˮc��ʣ��Na2SO3�ĺ���С��5mg/L������ˮb��NaClO�ĺ�����7.45mg/L������10m3��ˮb����������10%Na2SO3��Һ kg����Һ����仯���Բ��ƣ�
��15�֣���(1) 1�֣�����ÿ��2�֣�
��1��C ��2��ΪH2S�ȷֽⷴӦ�ṩ����
��3��ABC ��ֻдBC��1�֣������𰸲����֣�
��4��K2CO3��K2CO3��KOH��ֻ��KOH�����֣�
��5����Mg(OH)2
��2NH4++3Cl2+8OH- N2��+6Cl-+8H2O
N2��+6Cl-+8H2O
��SO42-��Ca2+ ��1.26
�������������
��1����H>0˵������Ӧ���ȣ�����Ӧ��ܴ��ڻ����169.8kJ��C��ȷ��A��B��������Ӧ��ܱ��淴Ӧ��ܴ�169.8kJ��D����
��2������ֽ�������������ͨ�����ʹ��������ȼ�����ṩ����ֽ�����������
��3��A��B�ֱ�ΪH��Oԭ�ӣ�����4000��~5000��ʱA��B��C������Ӧ���ܷ�����
��4��������ӦΪ2H2O+2e-=H2��+2OH-�����������˵�����������NΪ-3�ۣ���������ͬʱ���ɶ�����̼��������̼��KOH����̼��ء�����ų�Һ�е���Ҫ�ɷ���̼��ػ�̼��غ��������ء�
��5���ٵ�pH=11ʱ����Һ��c(Mg2+)=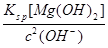 =5.6��10-6<10-5��Mg2+������ȫ����������a�к���������þ�����������������½�NH4+��������N2����������ԭΪCl-�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ����BaCO3(s)
=5.6��10-6<10-5��Mg2+������ȫ����������a�к���������þ�����������������½�NH4+��������N2����������ԭΪCl-�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ����BaCO3(s)  Ba2+(aq)+CO32-(aq)��SO42-��Ba2+��CO32-��Ca2+�γɸ����ܵ����ᱵ��̼��ƣ����Թ���II����̼�ᱵ��ȥCa2+��SO42-���ܹ���III��������������Һ��ԭ��Һ��ClO-���ɵ�ʧ������ȵ�n(ClO-)=n(SO32-)��
Ba2+(aq)+CO32-(aq)��SO42-��Ba2+��CO32-��Ca2+�γɸ����ܵ����ᱵ��̼��ƣ����Թ���II����̼�ᱵ��ȥCa2+��SO42-���ܹ���III��������������Һ��ԭ��Һ��ClO-���ɵ�ʧ������ȵ�n(ClO-)=n(SO32-)�� ��m(Na2SO3��Һ)=1.26kg��
��m(Na2SO3��Һ)=1.26kg��
���㣺 ��ѧ��Ӧ������ ��� ͼ��ķ��� �绯ѧ �ܶȻ� ���ӷ���ʽ ������ԭ��Ӧ ��ѧ����

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�ijʵ��С����0.50 mol��L-1NaOH��Һ��0.50 mol��L-1������Һ�����к��ȵIJⶨ��
��.����0.50 mol��L-1NaOH��Һ
(1)��ʵ���д�ԼҪʹ��470 mL NaOH��Һ,������Ҫ����NaOH������������g��
(2)��ͼ��ѡ�����NaOH��������Ҫ��������(����ĸ):����������
| ���� | ������ƽ (������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |
��.�ⶨ�к���
(1)ʵ�����ϱ����ձ�(��С�����ձ�)����ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����ᡢNaOH��Һ,��ȱ�ٵ�ʵ�鲣����Ʒ������������
(2)ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ��,ʵ���������±���
������д�±��еĿհ�:
| ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ (t2-t1)/�� | |||
| H2SO4 | NaOH | ƽ��ֵ | ||||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | | |
�ڽ�����Ϊ0.50 mol��L-1NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3,�кͺ�������Һ�ı�����c=��4.18��J/(g����)�����к��Ȧ�H=������������(ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ��mol-1��ƫ��,����ƫ���ԭ�������(����ĸ)����������
A.ʵ��װ�ñ��¡�����Ч����
B.��ȡNaOH��Һ�����ʱ���Ӷ���
C.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
 2NH3��g������Ӧ���̵������仯��ͼ��ʾ����֪N2��g����H2��g����Ӧ����17 g NH3��g�����ų�46.1 kJ��������
2NH3��g������Ӧ���̵������仯��ͼ��ʾ����֪N2��g����H2��g����Ӧ����17 g NH3��g�����ų�46.1 kJ��������
 H++ SO42-
H++ SO42-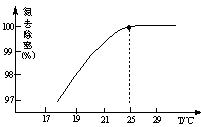
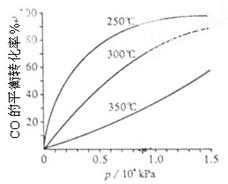
 2SO3(g) ?H=-196��6 kJ��mol-1
2SO3(g) ?H=-196��6 kJ��mol-1 2NO2(g) ?H=-113��0 kJ��mol-1
2NO2(g) ?H=-113��0 kJ��mol-1 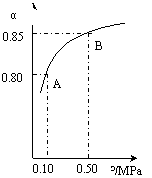


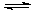 H++ SO42-
H++ SO42- �ķ�ˮ�ᷢ�����·�Ӧ��
�ķ�ˮ�ᷢ�����·�Ӧ��