��Ŀ����
��14�֣���֪��NaAlO2�Ʊ�����Al(OH)3������ת����ϵ��ͼ��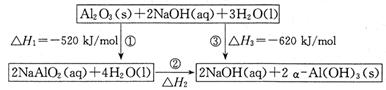
��1����Ӧ�ڵ��Ȼ�ѧ����ʽΪ ��
��2��������������ת����ϵ���ݶ���˾�ҵ���һ�ּ�ݵĴ��������ȡAl2O3�ķ������������£�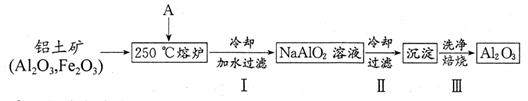
������A�Ļ�ѧʽΪ ��
�ڲ����Ļ�ѧ��Ӧ����ʽΪ �����鲽����г����Ƿ�ϴ���ķ����� ��
�۲���������ȴ�ķ�����������Al(OH)3���ô�ʩ�������� ��
�ܹ�ҵ�Ͽɵ����������Al2O3�Ի��Al�������2.7kgAl��������������A�����ʵ�������Ϊ mol���������������Na[AlCl4]��NaCl�Ļ�������Al2O3���е����Al����������ӦΪ ��
��14�֣�
��1��NaAlO2(aq)+2H2O(l) = NaOH(aq)+����Al(OH)3��s�� ?H=��50kJ?mol?1��2�֣�
��2����NaOH��2�֣� ��2Al(OH)3 Al2O3+3H2O��2�֣�
Al2O3+3H2O��2�֣�
ȡ���һ��ϴ��Һ����ɫ��Ӧ���������û�л�ɫ���Ѿ�ϴ������֮��δϴ����2�֣�
��NaAlO2ˮ�����ɦ���Al(OH)3Ϊ���ȷ�Ӧ����ȴ���´�ʹNaAlO2ˮ�������ƶ����С����Al(OH)3��ǿ���е��ܽ⣨2�֣�
��100��2�֣� AlCl4-+3e?=Al+4Cl?��2�֣�
���������������1������д����Ӧ�ڵĻ�ѧ����ʽ��ע��״̬NaAlO2(aq)+2H2O(l) = NaOH(aq)+����Al(OH)3��s�����ݸ�˹��������÷�Ӧ���ʱ�?H=��1/2?H1 + 1/2?H3=��50kJ?mol?1���ɵ��Ȼ�ѧ����ʽΪ��
��2����Al2O3Ϊ�����������������AΪNaOH��
�ڲ����ΪAl(OH)3���շֽ�����Al2O3�����Ի�ѧ����ʽΪ��2Al(OH)3 Al2O3+3H2O��������г������û��ϴ�Ӹɾ�������Ậ��Na+�����Լ��鷽��Ϊ��ȡ���һ��ϴ��Һ����ɫ��Ӧ���������û�л�ɫ���Ѿ�ϴ������֮��δϴ����
Al2O3+3H2O��������г������û��ϴ�Ӹɾ�������Ậ��Na+�����Լ��鷽��Ϊ��ȡ���һ��ϴ��Һ����ɫ��Ӧ���������û�л�ɫ���Ѿ�ϴ������֮��δϴ����
�۸��ݣ�1�����Ȼ�ѧ����ʽ��֪NaAlO2ˮ�����ɦ���Al(OH)3Ϊ���ȷ�Ӧ����ȴ���´�ʹNaAlO2ˮ�������ƶ����С����Al(OH)3��ǿ���е��ܽ⡣
�ܸ��ݻ�ѧ����ʽ�ɵö�Ӧ��ϵ��Al �� NaOH ����n��NaOH��=2700g��27g/mol=100mol�������Ϸ���AlCl4-�õ��ӷ�Ӧ���缫����ʽΪ��AlCl4-+3e?=Al+4Cl?
���㣺���⿼���Ȼ�ѧ����ʽ����д����ѧ���̵ķ��������ӵļ��顢����ʽ����д����ѧ���㡣

��������ȡ�봢��������Դ����������о��ȵ㡣
��1����֪��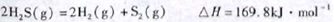
�����йظ÷�Ӧ��������ȷ����
| A������Ӧ���С��169.8kJ��mol-1 |
| B���淴Ӧ���һ��С��169.8kJ��mol-1 |
| C������Ӧ��ܲ�С��169.8kJ��mol-1 |
| D������Ӧ��ܱ��淴Ӧ���С169.8kJ��mol-1 |
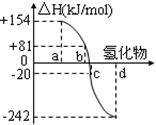
��3��H2O���ȷֽ�Ҳ�ɵõ�H2��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ����ͼ1��ʾ����4000��~5000��ʱ���ܷ���������Щ��Ӧ ����д��ĸ����
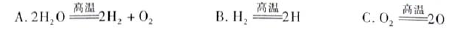
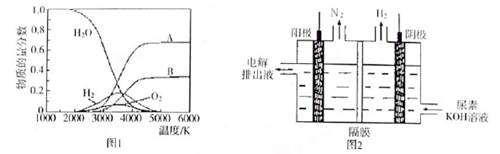
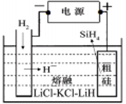
��4����ȡ��������һ�ַ����ǵ������[CO��NH2��2]�ļ�����Һ��װ��ʾ��ͼ��ͼ2�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�����õ��װ���е���ų�Һ�е���Ҫ�ɷ��� ��д��ѧʽ����
��5����֪�������ʵ�KSP��

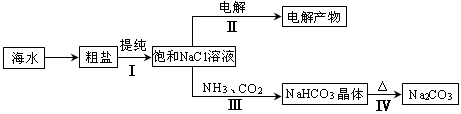
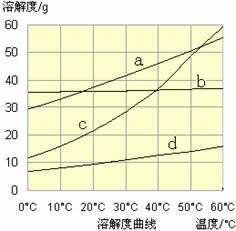
5.6��10-12��Ca��OH��2��1.4��10-5���ȼҵ�� ��ⱥ��ʳ��ˮҲ�ܵõ�������������õ���ˮ�辫�ƣ�ȥ����Ӱ���Ca2+��Mg2+��NH4+��SO42��[c��SO42����>c(Ca2+)]��ij�����������£�
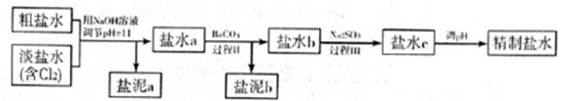
������a����ɳ�⣬�����е������� ��
�ڹ���I�н�NH4+ת��ΪN2�����ӷ���ʽ�� ��
�۹���II�г�ȥ�������� ��
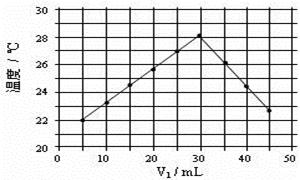
�ܾ�����III������������ˮc��ʣ��Na2SO3�ĺ���С��5mg/L������ˮb��NaClO�ĺ�����7.45mg/L������10m3��ˮb����������10%Na2SO3��Һ kg����Һ����仯���Բ��ƣ�
��7�֣�(1)���������ھ�������������ˮ������������ҵ�������ΪƯ����
�ٳ������������������ҿ������������е��ʷ�Ӧ���磺
6Ag(s)+O3(g)===3Ag2O(s)����H =" -235.8" kJ/mol;
��֪��2 Ag2O(s)===4Ag(s)+O2(g)����H = +62.2kJ/mol;
��Ӧ 2O3��g��= 3O2��g�� �ġ�H = kJ/mol��
�ڿ�ѧ��P��Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в�
�����������������������ɹ������⣬�����缫��ӦʽΪ �� ��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�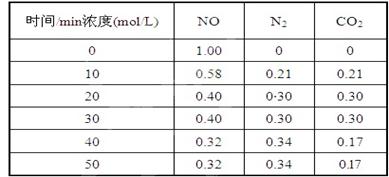
����10 min��20 min��ʱ����ڣ���CO2��ʾ�ķ�Ӧ����Ϊ ��
��д���÷�Ӧ��ƽ�ⳣ���ı���ʽK= ��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ��״̬���� (�������ĸ)��
| A��������ѹǿ���ֲ��� | B��2v��(NO)=v��(N2) |
| C��������CO2������������� | D�����������ܶȱ��ֲ��� |
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� (����������䡱��С��)��
 N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ��
N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ��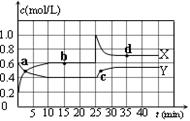
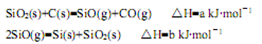
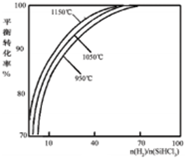
 Si��s��+3HCl��g����ͬ�¶ȼ���ͬn��H2��/n��SiHCl3��ʱ����Ӧ��X��ƽ��ת���ʹ�ϵ��ͼ��
Si��s��+3HCl��g����ͬ�¶ȼ���ͬn��H2��/n��SiHCl3��ʱ����Ӧ��X��ƽ��ת���ʹ�ϵ��ͼ��