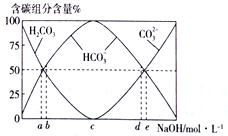
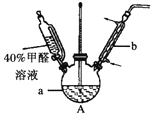
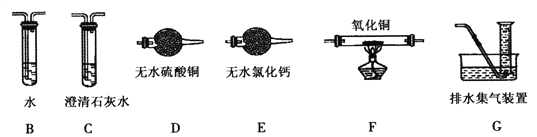
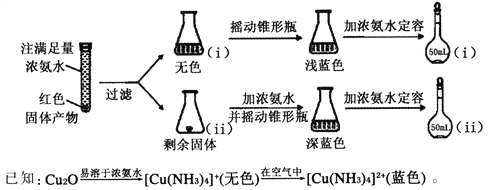
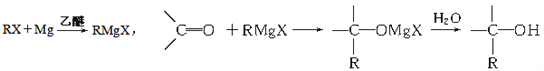��Ŀ����
����Ŀ��������ڡ����ɵĻ�ѧ�ҡ�һ����˵�����ڻ�ĵ��������£����������е�ˮ����ɥʧ��ԭ�е���ɫ�����һ�ֺ�ɫ���ʣ�������ʹ���κθ����Լ��������ֺ�ɫ����Ҳ���Եõ��������ù��̿ɱ�ʾΪ��![]() ������˵���������
������˵���������
A. ������������ı仯����������ԭ��Ӧ B. �˷�Ӧ�ɴ��Բⶨ�����������ĺ���
C. ������������ı仯��һ������Ļ�ѧ��Ӧ D. ���ȷֽⷨ�����������Ƶõ��ʹ�
���𰸡�C
��������A. ��Ӧ�����������Hg�������Ϸ�Ӧ��������������˲�����������ı仯����������ԭ��Ӧ��A��ȷ��B. ���ݷ�Ӧԭ����֪�˷�Ӧ�ɴ��Բⶨ�����������ĺ�����B��ȷ��C. �÷�Ӧ���ǿ��淴Ӧ��C����D. Hg�Dz����õĽ��������ȷֽⷨ�����������Ƶõ��ʹ���D��ȷ����ѡC��

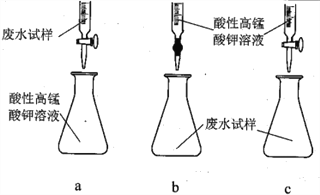
����Ŀ��ij�������ŷŵĹ�ҵ��ˮ����Ҫ��Na+��HSO3����SO42-���о�С�����ⶨ����HSO3����Ũ�ȣ������������������
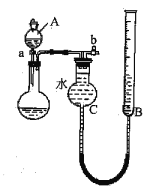
��ȡ20.00mL��ˮ��������0.02moL��K-1MnO4����Һ���еζ�����¼���ݣ����㡣
�ش���������
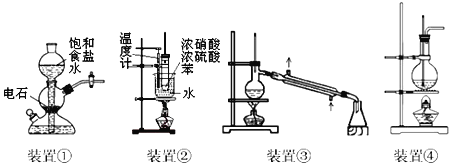
��1��������ͼ��ʾ��װ����ɷ���һ
������A��������______________��
�ڶ���ǰ��Ӧ���еIJ�����____________________________��
�۸÷������ڽϴ�������ܵ�һ��ԭ����____________________________��
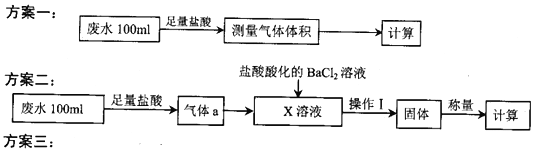
��2���ٷ���������������a����X��Һ��������______________ (����)��
a.Na2SO3��Һ b.˫��ˮ c.��������Һ d.H2SO4�ữ��KMnO4��Һ
������Ϊ�������ƣ�д������a��X��Һ��Ӧ�����ӷ���ʽ____________________________��
�۸÷����У�����I�����IJ�����������Ϊ______________��
��3���ٷ�������Ƶ����еζ���ʽ�У����������______________ (����)���÷����Ƿ���Ҫָʾ��? ______________ (����������������)��ԭ����__________________________________________��
�ڵζ���¼�������±���
�ζ�ǰ����/mL | �ζ������/mL | |
��һ�� | 0.10 | 16.12 |
�ڶ��� | 1.10 | 17.08 |
������ | 1.45 | 21.45 |
���Ĵ� | 0.00 | 16.00 |
����÷�ˮ������HSO3����Ũ��Ϊ______________ mol ��L-l��