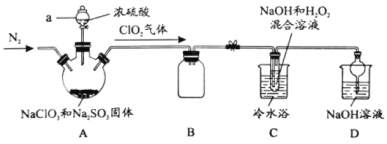
ЬтФПФкШн
ЁОЬтФПЁПЙ§бѕЛЏЧтЪЧживЊЕФЛЏЙЄВњЦЗЃЌЙуЗКгІгУгкЛЏбЇКЯГЩЁЂвНСЦЯћЖОЕШСьгђЁЃ
(1)Й§бѕЛЏЧтЕФЕчзгЪНЮЊ_____________ЁЃ
(2)ЙЄвЕЩЯЕчНтСђЫсЧтбЮШмвКЕУЕНЙ§ЖўСђЫсбЮ(![]() )ЃЌЙ§ЖўСђЫсбЮЫЎНтЩњГЩH2O2ШмвККЭСђЫсЧтбЮЃЌЩњГЩЕФСђЫсЧтбЮПЩвдбЛЗЪЙгУЁЃЕчНтСђЫсЧтбЮШмвКЪБбєМЋЕФЕчМЋЗДгІЪНЮЊ_______ЁЃаДГіЙ§ЖўСђЫсбЮЫЎНтЕФРызгЗНГЬЪН________ЁЃ
)ЃЌЙ§ЖўСђЫсбЮЫЎНтЩњГЩH2O2ШмвККЭСђЫсЧтбЮЃЌЩњГЩЕФСђЫсЧтбЮПЩвдбЛЗЪЙгУЁЃЕчНтСђЫсЧтбЮШмвКЪБбєМЋЕФЕчМЋЗДгІЪНЮЊ_______ЁЃаДГіЙ§ЖўСђЫсбЮЫЎНтЕФРызгЗНГЬЪН________ЁЃ
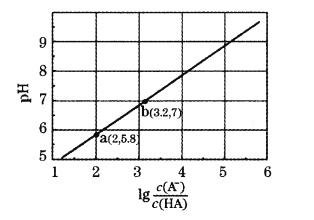
(3)298KЪБЃЌЪЕбщВтЕУЗДгІ![]() дкВЛЭЌХЈЖШЪБЕФЛЏбЇЗДгІЫйТЪШчБэЃК
дкВЛЭЌХЈЖШЪБЕФЛЏбЇЗДгІЫйТЪШчБэЃК
ЪЕбщБрКХ | 1 | 2 | 3 | 4 | |
c(HI) /molЉqL-1 | 0.100 | 0.200 | 0.300 | 00.100 | 0.100 |
c(H2O2)/molЉqL-1 | 0.100 | 0.100 | 0.100 | 0.200 | 0.300 |
v//molЉqL-1Љqs-1 | 0.007 60 | 0.015 3 | 0.022 7 | 0.015 1 | 0.022 8 |
вбжЊЫйТЪЗНГЬЮЊ![]() ЃЌЦфжаkЮЊЫйТЪГЃЪ§ЁЃ
ЃЌЦфжаkЮЊЫйТЪГЃЪ§ЁЃ
ИљОнБэжаЪ§ОнХаЖЯЃКa=_______ЃЌb=________ЁЃ
(4)ЁАДѓЯѓЕФбРИрЁБЪЕбщЪЧНЋХЈЫѕЕФЙ§бѕЛЏЧтгыЗЪдэвКЛьКЯЃЌдйЕЮМгЩйСПЕтЛЏМиШмвКЃЌМДПЩЙлВьЕНХнФзДЮяжЪЯёХчШЊвЛбљХчгПЖјГіЁЃЗДгІжаH2O2ЕФЗжНтЛњРэЮЊЃК
![]() Т§
Т§
![]() Пь
Пь
ДЫЗДгІЙ§ГЬжаЮоДпЛЏМСКЭгаДпЛЏМСЕФФмСПБфЛЏЙиЯЕЭМЯёШчЭМЫљЪОЃК
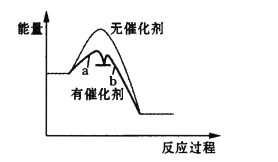
дђБэЪОТ§ЗДгІЕФЧњЯпЪЧ__________(ЬюЁАaЁБЛђЁАbЁБ)ЁЃ
1mol H2O2ЗжНтЗХГіШШСП98 kJЃЌдђH2O2ЗжНтЕФШШЛЏбЇЗНГЬЪНЮЊ_________________ЁЃ
(5)ФГПЦбаЭХЖгбаОП![]() ЬхЯЕ(Цфжа
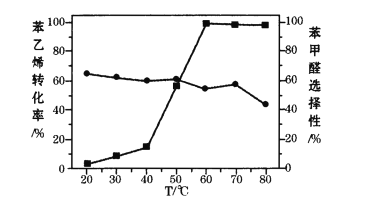
ЬхЯЕ(Цфжа![]() )бѕЛЏБНввЯЉжЦШЁБНМзШЉЃЌЗДгІЕФИБВњЮяжївЊЮЊБНМзЫсКЭЛЗбѕБНввЭщЁЃвЛЖЈЬѕМўЯТЃЌВтЕУвЛЖЈЪБМфФкЮТЖШЖдбѕЛЏЗДгІЕФгАЯьШчЭМЃК
)бѕЛЏБНввЯЉжЦШЁБНМзШЉЃЌЗДгІЕФИБВњЮяжївЊЮЊБНМзЫсКЭЛЗбѕБНввЭщЁЃвЛЖЈЬѕМўЯТЃЌВтЕУвЛЖЈЪБМфФкЮТЖШЖдбѕЛЏЗДгІЕФгАЯьШчЭМЃК
зЂЃКЁіБНввЯЉзЊЛЏТЪ ЁёБНМзШЉбЁдёад
Ђй80ЁцЪББНввЯЉЕФзЊЛЏТЪгаЫљНЕЕЭЃЌЦфдвђПЩФмЪЧ_______ЁЃ
ЂкНсКЯБНввЯЉЕФзЊЛЏТЪЃЌвЊЛёЕУНЯИпЕФБНМзШЉВњТЪЃЌгІИУбЁдёЕФЮТЖШЮЊ_______ЁЃ
ЁОД№АИЁП![]() 2HSO
2HSO![]() -2e-=S2O
-2e-=S2O![]() +2H+Лђ2SO
+2H+Лђ2SO![]() -2e-=S2O
-2e-=S2O![]() S2O
S2O![]() +2H2O=2HSO
+2H2O=2HSO![]() +H2O2ЛђS2O
+H2O2ЛђS2O![]() +2H2O =2H++SO
+2H2O =2H++SO![]() +H2O2 1 1 a 2H2O2(l)=2H2O(l)+O2(g) ЁїH=-196kJЁЄmol-1 ИпЮТДйНјСЫЙ§бѕЛЏЧтКЭЙ§бѕввЫсЗжНт 70Ёц
+H2O2 1 1 a 2H2O2(l)=2H2O(l)+O2(g) ЁїH=-196kJЁЄmol-1 ИпЮТДйНјСЫЙ§бѕЛЏЧтКЭЙ§бѕввЫсЗжНт 70Ёц
ЁОНтЮіЁП
(1)Й§бѕЛЏЧтЮЊЙВМлЛЏКЯЮяЃЌУПИібѕдзггыСэвЛИібѕдзгЙВгУвЛЖдЕчзгЃЌгывЛИіЧтдзгЙВгУвЛЖдЕчзгЃЌЫљвдЕчзгЪНЮЊ![]() ЃЛ
ЃЛ
(2)ЕчНтГижабєМЋЗЂЩњбѕЛЏЗДгІЃЌИљОнЬтвтПЩжЊЕчНтЪБСђЫсИљ(СђЫсЧтИљ)БЛбѕЛЏГЩS2O![]() ЃЌЫљвдбєМЋЗДгІЪНЮЊ2HSO
ЃЌЫљвдбєМЋЗДгІЪНЮЊ2HSO![]() -2e-=S2O
-2e-=S2O![]() +2H+Лђ2SO
+2H+Лђ2SO![]() -2e-=S2O
-2e-=S2O![]() ЃЛЙ§ЖўСђЫсбЮЫЎНтЩњГЩЋYH2O2ШмвККЭСђЫсЧтбЮЃЌЫљвдЫЎНтРызгЗНГЬЪНЮЊS2O
ЃЛЙ§ЖўСђЫсбЮЫЎНтЩњГЩЋYH2O2ШмвККЭСђЫсЧтбЮЃЌЫљвдЫЎНтРызгЗНГЬЪНЮЊS2O![]() +2H2O=2HSO
+2H2O=2HSO![]() +H2O2ЛђS2O
+H2O2ЛђS2O![]() +2H2O =2H++SO
+2H2O =2H++SO![]() +H2O2ЃЛ
+H2O2ЃЛ
(3)ИљОнБэИёЪ§ОнЕУЃК0.0076=kЁС0.100aЁС0.100bЃЌ0.0153=kЁС0.100aЁС0.200bЃЌ0.0151=kЁС0.200aЁС0.100bЃЌСЊСЂПЩЕУ2a=1ЃЌ2b=1ЃЌЫљвдa=1ЃЌb=1ЃЛ
(4)ЗДгІЕФЛюЛЏФмдНДѓЗДгІЫйТЪдНТ§ЃЌОнЭМПЩжЊaЧњЯпЫљЪОЕФЗДгІЛюЛЏФмНЯДѓЃЌЫљвдЧњЯпaДњБэЕФЪЧТ§ЗДгІЃЛ
1mol H2
(5)ЂйИпЮТДйНјСЫЙ§бѕЛЏЧтКЭЙ§бѕввЫсЗжНтЃЌЕМжТБНввЯЉЕФзЊЛЏТЪгаЫљНЕЕЭЃЛ
ЂкОнЭМПЩжЊ60ЁцвдЩЯЪББНввЯЉЕФзЊЛЏТЪНгНќ100%ЃЌЖјЮТЖШИпгк60ЁцЪБЃЌ70ЁцЪББНМзШЉЕФбЁдёадзюДѓЃЌЫљвдЗДгІЮТЖШгІбЁ70ЁцЁЃ

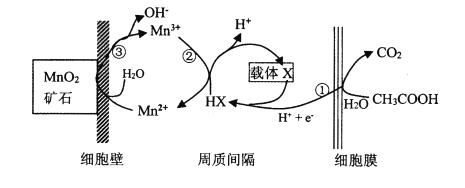
 гЅХЩНЬИЈЯЮНгНЬВФКгББНЬг§ГіАцЩчЯЕСаД№АИ
гЅХЩНЬИЈЯЮНгНЬВФКгББНЬг§ГіАцЩчЯЕСаД№АИ ГѕжаЪюЦкЯЮНгЯЕСаД№АИ
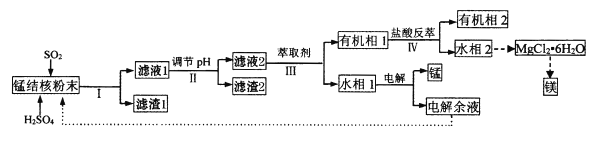
ГѕжаЪюЦкЯЮНгЯЕСаД№АИЁОЬтФПЁПгУКЃЕзУЬНсКЫ(жївЊГЩЗжЮЊMnO2ЃЌКЌЩйСПMgOЁЂFe2O3ЁЂAl2O3ЁЂSiO2)ЮЊдСЯЃЌжЦБИН№ЪєУЬЁЂУОЕФвЛжжЙЄвеСїГЬТЗЯпШчЯТЃК
вбжЊЃКЂйМИжжФбШмЮяЕФШмЖШЛ§(25Ёц)ШчЯТБэЫљЪОЃК
ЛЏбЇЪН | Mg(OH)2 | Mn(OH)2 | Al(OH)3 | Fe(OH)3 |
Ksp | 1.8ЁС10-11 | 1.8ЁС10-13 | 1.0ЁС10-33 | 4.0ЁС10-38 |
ЂкШмвКжаФГРызгХЈЖШЁм1.0ЁС10-6molЁЄL-1ЪБЃЌШЯЮЊИУРызгГСЕэЭъШЋЁЃ
ЭъГЩЯТСаЮЪЬтЃК
(1)ЁАУЬНсКЫЗлФЉЁБжаMnO2гыSO2ЗДгІЕФРызгЗНГЬЪНЮЊ_____ЁЃ
(2)ЁАТЫвК1ЁБжаc(Mn2+)ЮЊ0.18molЁЄL-1ЃЌдђЁАЕїНкpHЁБЕФЗЖЮЇЮЊ_______ЃЌЁАТЫдќ2ЁБЕФГЩЗжЮЊ______ЁЃ
(3)ЁАЂѓЁБДІЁАнЭШЁМСЁБнЭШЁЕФГЩЗжЪЧ___ЃЛЁАЂєЁБДІгУЁАбЮЫсЗДнЭЁБЕФзїгУЪЧ____ЁЃ
(4)MgCl2ЁЄ6H2OжЦШЁЮоЫЎMgCl2ЪБЃЌашвЊдкИЩдяЕФHClЦјСїжаМгШШЗжНтЁЃHClЕФзїгУЮЊ__________ЁЃ
(5)ИУЙЄвеСїГЬжаГ§ЕчНтгрвКПЩбЛЗРћгУЭтЃЌЛЙФмбЛЗРћгУЕФЪдМСЮЊ____ЁЃ
(6)вЛжжКЃЩњМйЕЅцпОњ(ВЩздЬЋЦНбѓЩюКЃДІ)ЃЌдкЮобѕЬѕМўЯТвдДзЫсЮЊЕчзгЙЉЬхЛЙдMnO2ЩњГЩMn2+ЕФЛњРэШчЭМЫљЪОЁЃаДГіЗДгІЂкЂлЕФзмЗДгІЕФРызгЗНГЬЪН____________ЁЃ