��Ŀ����
����Ŀ���ش��������⣺
��1��25��ʱ��pH��3����������ˮ�������c(H��)Ϊ_______��
��2��99��ʱ����pH��6������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��pH��2����ʱˮ�����ӻ�Kw��_____����Һ��c(OH-)Ϊ_____��
��3��25��ʱ��a mol��L��1CH3COOH��Һ��pH��b���ú�a��b�Ĵ���ʽ��ʾCH3COOH�ĵ���ƽ�ⳣ��Ka��________��
��4��25��ʱ��pH��3�Ĵ����pH��11��NaOH��Һ�������Ϻ���Һ��___(������������������������)�ԡ�
��5��25��ʱ��Ka(CH3COOH)��1.8��10��5��Ka(HSCN)��0.13�����¶��½�20mL 0.1mol��L��1CH3COOH��Һ��20mL 0.1mol��L��1HSCN��Һ�ֱ���20mL 0.1mol��L��1NaHCO3��Һ��ϣ�ʵ���ò������������(V)��ʱ��(t)�仯��ʾ��ͼ��ͼ��ʾ����Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ����_____________________________________________��

���𰸡�10-11 mol��L��1 10-12 10-10 mol��L��1 ![]() ��
��![]() �� ����K(CH3COOH)<K(HSCN)������ͬ�¶ȡ���ͬ���ʵ���Ũ�Ⱥ����ʱ��HSCN��Һ�е�c(H+)����CH3COOH��Һ�е�c(H+)������NaHCO3��Ӧ���ʴ�
�� ����K(CH3COOH)<K(HSCN)������ͬ�¶ȡ���ͬ���ʵ���Ũ�Ⱥ����ʱ��HSCN��Һ�е�c(H+)����CH3COOH��Һ�е�c(H+)������NaHCO3��Ӧ���ʴ�
��������
(1)������������������ˮ�ĵ��룬��������������������ˮ�ĵ��룬���c(OH-)=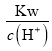 ���㣻
���㣻
(2)��pH=6������ˮ��֪�����¶���ˮ���������c(H+)=c(OH-)=1.0��10-6 mol/L�����Kw=c(OH-)��c(H+)����ˮ�����ӻ���NaHSO4�������������������ˮ�ĵ��룬��Һ����������������ˮ�ĵ��룻����ԭ���غ������
(3)����pH��b����֪c(H+)=1.0��10-b mol/L�����CH3COOH![]() H++CH3COO-��
H++CH3COO-��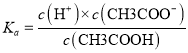 ���㣻
���㣻
(4)����Ϊ���ᣬ���Һ�д����������Һ�����ԣ���ϵ���غ������
(1)��������������������ˮ�ĵ���,��25��CʱpH=3��������ˮ�������c(OH-)=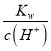 =
=![]() =10-11mol/L��
=10-11mol/L��
(2)��pH=6������ˮ��֪�����¶���ˮ���������c(H+)=c(OH-)=1.0��10-6 mol/L����ʱKw=c(OH-)c(H+)=1.0��10-12�� NaHSO4�������������������ˮ�ĵ�����Һ����������������ˮ�ĵ��룬��ˮ���������c(OH-)=![]() ���ʴ�Ϊ1.0��10-12��10-10 mol��L��1��
���ʴ�Ϊ1.0��10-12��10-10 mol��L��1��
(3)����pH��b����֪c(H+)=1.0��10-bmol.L-1����CH3COOH![]() H++CH3COO-��֪
H++CH3COO-��֪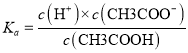 =
=![]() =
=![]() ���ʴ�Ϊ
���ʴ�Ϊ![]() ��
��![]() ��
��
(4)CH3COOHΪ���ᣬpH��3�Ĵ����pH��11��NaOH��Һ�������Ϻ�CH3COOH��������Һ�����ԣ���c(H+)>c(OH-)���ʴ�Ϊ���
(5)�������֪��Ka(CH3COOH) <Ka(HSCN)������ͬ�¶ȡ���ͬ���ʵ���Ũ�Ⱥ����������Һ�У�HSCN��Һ�е�c(H+)����CH3COOH��Һ�е�c(H+)���ʷ�Ӧ��ʼ�Σ�HSCN��NaHCO3��Ӧ���ʴʴ�Ϊ������K(CH3COOH)<K(HSCN)������ͬ�¶ȡ���ͬ���ʵ���Ũ�Ⱥ����ʱ��HSCN��Һ�е�c(H+)����CH3COOH��Һ�е�c(H+)����NaHCO3��Ӧ���ʴ�