��Ŀ����
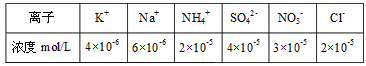
����Ŀ�����������г���MnS����������ȥ��ҵ��ˮ�е�Cu2����Cu2��(aq)��MnS(s)![]() CuS(s)��Mn2��(aq)�����й���������ȷ����(����)
CuS(s)��Mn2��(aq)�����й���������ȷ����(����)
��MnS��Ksp����CuS��Ksp���ڴﵽƽ��ʱc(Mn2��)��c(Cu2��)������Һ�м�������Na2S�������Һ��c(Cu2��)��c(Mn2��)���ֲ��䡡�ܸ÷�Ӧƽ�ⳣ��K��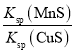
A. �٢� B. �ڢ�
C. �٢ڢ� D. �ڢۢ�
���𰸡�A
����������ѧʽ���Ƶ����ʣ��ܶȻ��������ת��Ϊ�ܶȻ�С�ģ�����MnS��Ksp��CuS��Ksp����ȷ���÷�Ӧ�ﵽƽ��ʱ�����ӵ�Ũ�ȱ��ֲ��䣬����һ����ȣ��ڴ�����Һ�м�������Na2S�������Һ��c(S2��)���������¶Ȳ��䣬�ܶȻ����䣬c(Cu2��)��c(Mn2��)����С���۴���Ӧ��ƽ�ⳣ��K��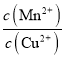 ��
��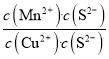 ��
��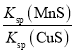 ������ȷ�����ϣ���ѡA��
������ȷ�����ϣ���ѡA��

��ϰ��ϵ�д�
 һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�
�����Ŀ