��Ŀ����
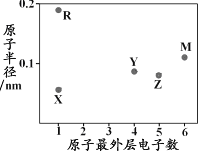
����Ŀ��������Ԫ��X��Y��Z��ԭ������������������ЩԪ����ɵ����ֳ���10������A��B��C��D��ת����ϵ��ͼ��ʾ(������ʡ��)������B��D�����Ԫ����ͬ��DΪ������ɫҺ�塣�����й�˵��һ���������( )

A.��AΪ�⻯������ȶ��ԣ�A>D
B.��AΪ�⻯�������Ӱ뾶��Z>Y
C.��CΪ�⻯����۷е㣺C<D
D.��CΪ�⻯���X��Z�γɵĻ������п��ܴ��ڷǼ��Լ�
���𰸡�B
��������
DΪ������ɫҺ����Ϊ10����������DΪH2O��B��D(H2O)�����Ԫ����ͬ����BΪOH-��H3O+��
��BΪOH-����AΪHF��NH4+��CΪF-��NH3����X��Y��Z�ֱ�ΪH��O��F��H��N��O��
��BΪH3O+����AΪNH3��CΪNH4+����X��Y��Z�ֱ�ΪH��N��O���ݴ˽��
A����AΪ�⻯���AΪHF��DΪH2O�����ڷǽ����ԣ�F��O�������ȶ��ԣ�A(HF)��D(H2O)��A��ȷ��
B����AΪ�⻯��(HF)����ZΪF��YΪO��F-��O2-������ͬ�ĵ��Ӳ�ṹ��F-�ĺ˵������O2-�ĺ˵�����ʰ뾶��Z(F-)��Y(O2-)����AΪ�⻯�NH3������YΪN��ZΪO��N3-��O2-������ͬ�ĵ��Ӳ�ṹ��N3-�ĺ˵������O2-�ĺ˵����С���ʰ뾶��Z(O2-)<Y(N3-)��B����
C����CΪ�⻯���CΪNH3��DΪH2O���е�C(NH3)��D(H2O)��C��ȷ��
D����CΪ�⻯���XΪH��ZΪO����X��Z�γɵĻ������п��ܴ��ڷǼ��Լ�����H2O2��D��ȷ��
��ѡB��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���̵ĺϽ��仯�������ִ����������������Ź㷺��Ӧ�á��������������Ҫ�����������������ɵ���е�ȥ����������������Ҫ����Ԫ�ط��ϡ�
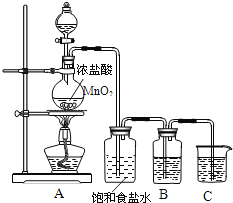
��ij���������̿�(MnCO3)[��FeCO3��SiO2��Cu2(OH)2CO3������]Ϊԭ�����������������̡������̾���Ĺ�����������ͼ��ʾ��

�����¼��ֽ������������ʱ��pH��
Mn(OH)2 | Fe(OH)3 | Cu(OH)2 | |
��ʼ����ʱ��pH | 7.2 | 2.7 | 4.7 |
��ȫ����ʱ��pH | x | 3.7 | 6.7 |
(1)�ù�����pHʱ��ѡ���Լ�a�ijɷ������________��������ijɷ���________������X��________��ϴ�ӡ����º�ɡ�
(2)Ϊȷ���������ʶ��ֲ���ʧ�̣�pH�����ķ�ΧΪ________��������������(X)������ȫ�ı���c(X)<1.0��10-5mol��L��Ksp[Mn(OH)2]=1.0��10-13����ʹMn2+��ȫ����ʱ��Һ��pH��СΪ________��
(3)����Һ�������ԣ���д����ⷨ�Ʊ�MnO2ʱ�����ϵĵ缫��Ӧʽ��________��
��50.7g MnSO4��H2O��Ʒ���ȷֽ���̵���������(��Ʒ�������¶ȱ仯����)����ͼ��ʾ��(��֪��M(Mn)=55g��mol)
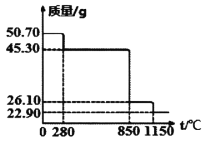
(1)850��ʱ�����ù���Ļ�ѧʽΪ��________________________��
(2)1150��ʱ����Ӧ�Ļ�ѧ����ʽΪ��________________________��