��Ŀ����
����Ŀ��������Ϊ�����Դ���Ź㷺��Ӧ��ǰ����������Ȼ���Ʊ��������������¡�
![]()
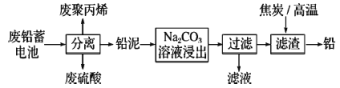
��ش��������⣺
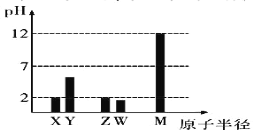
��.����ת�����ڴ����������£�ˮ������CH4���������ͼ����Ϣ�ش����⡣
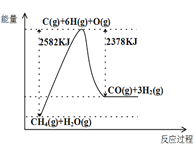
(1)�ù��̵��Ȼ�ѧ����ʽ��__________��
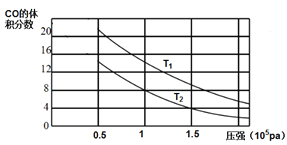
(2)ƽ��������CO�����������ѹǿ�Ĺ�ϵ��ͼ��ʾ���ж�T1��T2�Ĵ�С��ϵ��T1_______T2(����>����<������=��)����˵������__________��
(3)һ���¶��£���1L���ݵ��ܱ������г���1mol CH4��1molˮ������ַ�Ӧ��ƽ���÷�Ӧǰ����������������ʵ���֮����3:4������������·�Ӧ��ƽ�ⳣ��Ϊ______________��
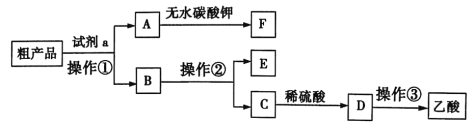
��.CO�任��500��ʱ��CO��һ����ˮ��Ӧ����CO2��H2��
��.ģ��H2�ᴿ���գ���CO2��H2����õ�H2�Ĺ������£�
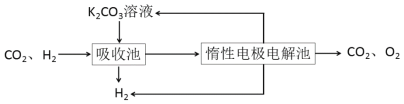
����ͼʾ��Ϣ�ش�
(4)���ճ��з�����Ӧ�����ӷ���ʽ��_________��
(5)д�����������������ĵ缫��Ӧʽ________����ϻ�ѧ����˵��K2CO3��Һ������ԭ��_________��
���𰸡�![]() > �����Ȼ�ѧ����ʽ
> �����Ȼ�ѧ����ʽ![]() ��֪�������¶ȣ�ƽ�������ƶ��� CO��������������ٶ�Ӧͼ��ѹǿһ��ʱ����T1>T2 0.75
��֪�������¶ȣ�ƽ�������ƶ��� CO��������������ٶ�Ӧͼ��ѹǿһ��ʱ����T1>T2 0.75 ![]()
![]() �����ŵ緢����Ӧ��
�����ŵ緢����Ӧ��![]() ������OH-����Һ�е�̼�����������Ӧ��
������OH-����Һ�е�̼�����������Ӧ��![]() ��ʵ����K2CO3��Һ������
��ʵ����K2CO3��Һ������
��������
��.(1)��ͼ��֪����ӦΪ���ȷ�Ӧ����H=2582kJ/mol-2378kJ/mol=+204kJ/mol���ݴ���д�Ȼ�ѧ����ʽ��
(2)����ѹǿ���¶ȶԻ�ѧƽ���Ӱ�������
(3)������ʽ����������������·�Ӧ��ƽ�ⳣ����
��. ��ͼ��֪�����ճ��ж�����̼��̼�����Һ�ķ�Ӧ�õ�̼����أ��ݴ���д���ӷ���ʽ��
��.(1) ��ͼ��֪��1mol���顢1molˮ��������1molCO��3mol������������(25822378)kJ=204kJ�����Ȼ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)�÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���������ͬѹǿ�£��¶�Խ�ߣ�CO���������Խ��T1>T2���ʴ�Ϊ��>�������Ȼ�ѧ����ʽ![]() ��֪�������¶ȣ�ƽ�������ƶ��� CO��������������ٶ�Ӧͼ��ѹǿһ��ʱ����T1>T2��
��֪�������¶ȣ�ƽ�������ƶ��� CO��������������ٶ�Ӧͼ��ѹǿһ��ʱ����T1>T2��
(3) һ���¶��£���1L���ݵ��ܱ������г���1molCH4��1mol��ˮ������ַ�Ӧ��ƽ���÷�Ӧǰ����������������ʵ���֮����3:4�������ļ������ʵ���x���ݴ˼��㣺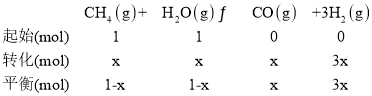
![]() ��x=
��x=![]() mol����ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊ��c(CH4)=c(H2O)=
mol����ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊ��c(CH4)=c(H2O)=![]() mol/L��c(CO)=
mol/L��c(CO)=![]() mol/L��c(H2)=1mol/L����÷�Ӧ��ƽ�ⳣ��K=
mol/L��c(H2)=1mol/L����÷�Ӧ��ƽ�ⳣ��K=![]() =0.75���ʴ�Ϊ��0.75��
=0.75���ʴ�Ϊ��0.75��
��.(4)��ͼ��֪�����ճ���������δ��Ӧ��������̼�����ս�����Ե缫����,�����ճ���Ϊ������̼��̼�����Һ�ķ�Ӧ�����ӷ���ʽΪ��CO32-+CO2+H2O=2HCO3-���ʴ�Ϊ��CO32-+CO2+H2O=2HCO3-��
(5)HCO3���ڵ���ƽ�⣺HCO3H++CO32������H+�ŵ�Ũ�ȼ�Сƽ�����ƣ�CO32-������������Ӧ��2H2O��2e��=H2����OH-��������Ӧ��![]() ��HCO3-+OH-�TCO32-+H2O��ʹ̼�����Һ�����������ʴ�Ϊ��
��HCO3-+OH-�TCO32-+H2O��ʹ̼�����Һ�����������ʴ�Ϊ��![]() �������ŵ緢����Ӧ��
�������ŵ緢����Ӧ��![]() ������OH-����Һ�е�̼�����������Ӧ��
������OH-����Һ�е�̼�����������Ӧ��![]() ��ʵ����K2CO3��Һ��������
��ʵ����K2CO3��Һ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�