��Ŀ����
��7�֣���ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��. SO2��2H2O��I2===H2SO4��2HI
��. 2HI H2��I2
H2��I2
��. 2H2SO4===2SO2��O2��2H2O
(1) ����������Ӧ�������ж���ȷ����_______________
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�����̲���1 mol O2��ͬʱ����1 mol H2
(2) һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��

�� 0��2 min�ڵ�ƽ����Ӧ����v(HI)��________________
�� ���¶��£�H2(g)��I2(g) 2HI(g)��ƽ�ⳣ��K��__________________
2HI(g)��ƽ�ⳣ��K��__________________
�� ��ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������_____________��ԭ����2����
a��ƽ�ⳣ�� b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ�� d��ƽ��ʱH2���������
(3) ʵ������Zn��ϡ������ȡH2����Ӧʱ���������������Լ��е�____________����H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
��. SO2��2H2O��I2===H2SO4��2HI
��. 2HI
 H2��I2
H2��I2��. 2H2SO4===2SO2��O2��2H2O
(1) ����������Ӧ�������ж���ȷ����_______________
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�����̲���1 mol O2��ͬʱ����1 mol H2
(2) һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��

�� 0��2 min�ڵ�ƽ����Ӧ����v(HI)��________________
�� ���¶��£�H2(g)��I2(g)
 2HI(g)��ƽ�ⳣ��K��__________________
2HI(g)��ƽ�ⳣ��K��__________________�� ��ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������_____________��ԭ����2����
a��ƽ�ⳣ�� b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ�� d��ƽ��ʱH2���������
(3) ʵ������Zn��ϡ������ȡH2����Ӧʱ���������������Լ��е�____________����H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
��1��C(1��)��2����0.1mol/(L.min)��2�֣� ��64 ��2�֣� ��b ��1�֣���3��b��1�֣�
�����������1��H2SO4�ڳ����£����ȶ����ֽ⣬���dz�ʶ������a�Ǵ���ģ���Ӧ����SO2�ǻ�ԭ����HI�ǻ�ԭ�����˻�ԭ��SO2��HI������b����ȷ������͢�ֱ����2�͢���ӵã�2H2O==2H2+O2������c��ȷ��d�Ǵ���ģ���ѡc��
��2������Ϊ �� (H2)="0." 1mol/1L/2min="0.05" mol��L-1��min-1�����Ը��ݷ�Ӧ����֮������Ӧ�Ļ�ѧ������֮�ȿ�֪���� (HI)="2" �� (H2)="0.1" mol��L-1��min-1��
�� 2HI(g)==H2(g)+I2(g)
2 1 1
��ʼŨ��/mol��L��1 1 0 0
�仯Ũ��/mol��L��1�� 0.2 0.1 0.1
ƽ��Ũ��/mol��L��1�� 0.8 0.1 0.1
���Ըÿ��淴Ӧ��ƽ�ⳣ��K=
 =64mol/L��
=64mol/L���������ʼʱ����HI������������2����������ƽ��״̬��ԭƽ��״̬����ǵȱ��ƻ������HI��H2��I2�����ʵ�����ƽ��Ũ�ȶ���ԭ����������������ֵİٷֺ��������������ƽ�ⳣ�����Dz���ġ����ڿ�ʼʱ��Ũ�������ˣ���Ӧ���ʼӿ죬��ƽ���ʱ�䲻������ԭ�������������Դ�ѡb��
��3��ˮ��������ʣ����ڵ���ƽ��H2O=H����OH�������������������Ӷ�ˮ�ĵ������������ã���п�����������Ӻ�������Ũ�Ƚ��ͣ��ٽ�ˮ�ĵ��룻������������ƣ�����Һϴ�൱����������Һ����ʱ����������������������������ƻ�������ӷ�Ӧ������������Ũ�ȣ���Ӧ���ʽ��ͣ������Ƶļ���Է�Ӧ��������Ӱ��ģ���������ͭ��п���û�����Cu����ԭ��أ��ӿ��˷�Ӧ���ʣ����Դ�ѡb��
������������2010��ɽ����28�⣬���������濼���ˡ��ܹ���ʵ������ֽ⣬ͨ���������֪ʶ�����÷������ۺϵķ��������ѧ����������������ܹ��������������Ĺ��̺ͳɹ�����ȷ�Ļ�ѧ���P���֡�ͼ����ģ�͡�ͼ�εȱ�����������͵������������������˿����Ƚ�İ�����龰����������ѭ���ֽ�ˮ���⡱���Կ�������������Ҫ��ϸߡ�

��ϰ��ϵ�д�
�����Ŀ
 Si(s)��3HCl(g)��
Si(s)��3HCl(g)��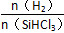 ʱ����Ӧ��X��ƽ��ת���ʹ�ϵ��ͼ��ʾ��
ʱ����Ӧ��X��ƽ��ת���ʹ�ϵ��ͼ��ʾ��
 CO(g) + 3H2(g) ��H =" +206.2" kJ/mol
CO(g) + 3H2(g) ��H =" +206.2" kJ/mol






 2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ�����
2NH3(g) ��H��0��������������ʱ�����¶ȣ���Ӧ���ʣ�(H2)��������ƽ��ת���ʾ����� 1/2N2O4��g�� ��H =��26��35 kJ��mol��1
1/2N2O4��g�� ��H =��26��35 kJ��mol��1
 H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���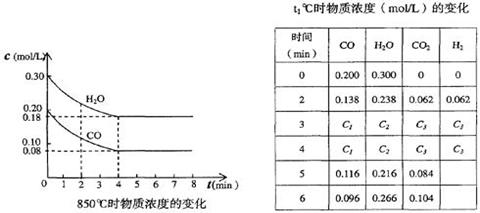
 1/2N2O4(g) ��H = ��26.35 kJ��mol��1
1/2N2O4(g) ��H = ��26.35 kJ��mol��1
 H2(g)��CO2(g)���÷�Ӧ��ƽ�ⳣ�����¶ȵı仯���±���
H2(g)��CO2(g)���÷�Ӧ��ƽ�ⳣ�����¶ȵı仯���±���