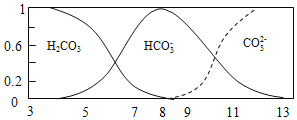
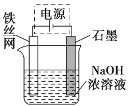
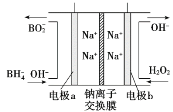
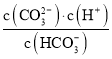��Ŀ����
����Ŀ�����ӵ�ص�Ӧ�úܹ㣬���������Ͽ��������á�ij���ӵ�����������������(LiCoO2)���������Ȳ�ں������ȡ����ʱ��������ӵ�ظ��������ķ�ӦΪ��6C+xLi++xe-��LixC6�������������¹������̻������������е�ijЩ������Դ(��������ĩ����)��
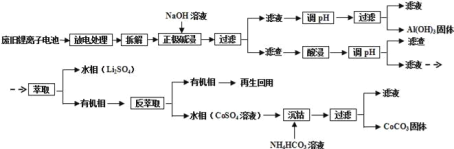
(1) LiCoO2�У�CoԪ�صĻ��ϼ�Ϊ____________��
(2)д��������������з�����Ӧ�����ӷ���ʽ_____________________________________��
(3)����H2SO4��H2O2�Ļ��Һ�������������һ����80���½��У�д���ò����з���������������ԭ��Ӧ�Ļ�ѧ����ʽ:__________________________________________�������������H2SO4��H2O2�Ļ��Һ����ȱ����___________________________��
(4)д�������ܡ������з�����Ӧ�Ļ�ѧ����ʽ_________________________________��
(5)��ŵ�����У�����LiCoO2��LixCoO2֮ͬ��ת����д���ŵ�ʱ��ط�Ӧ����ʽ_________.
���𰸡�+3 2Al+2OH-+2H2O=2AlO2-+3H2�� 2LiCoO2+H2O2+3H2SO4![]() Li2SO4+2CoSO4+O2��+4H2O ���������ɣ���Ⱦ�ϴ��������������ʣ��ں������������Է��� CoSO4+2NH4HCO3=CoCO3��+(NH4)2SO4+CO2��+H2O Li1-xCoO2+LixC6=LiCoO2+6C
Li2SO4+2CoSO4+O2��+4H2O ���������ɣ���Ⱦ�ϴ��������������ʣ��ں������������Է��� CoSO4+2NH4HCO3=CoCO3��+(NH4)2SO4+CO2��+H2O Li1-xCoO2+LixC6=LiCoO2+6C
��������
��1�����ݻ������У����ϼ۵Ĵ�����Ϊ0֪��LiCoO2�У�CoԪ�صĻ��ϼ�Ϊ+3�ۣ�
��2�������к���������������ǿ����Һ����AlO2-��
��3�����ʱ��Ӧ�������ᡢ���������Լ�LiCoO2����������Li2SO4��CoSO4����������Ϣ֪LiCoO2����ǿ�����ԣ�������������Ⱦ�������������ɣ����������������ʣ��ں������������Է��룻
��4���������������������ܺ�̼����立�Ӧ����̼���ܳ���������李�������̼��ˮ��
��5����ŵ�����У�Li1-xCoO2��LixC6����������ԭ��Ӧ����LiCoO2��C��
��1�����ݻ������У����ϼ۵Ĵ�����Ϊ0֪��LiCoO2�У�CoԪ�صĻ��ϼ�Ϊ+3�ۣ��ʴ�Ϊ��+3��
��2�������к���������������ǿ����Һ����AlO2-����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��3�����ʱ��Ӧ�������ᡢ���������Լ�LiCoO2����������Li2SO4��CoSO4����Ӧ����ʽΪ��2LiCoO2+H2O2+3H2SO4![]() Li2SO4+2CoSO4+O2��+4H2O����������Ϣ֪LiCoO2����ǿ�����ԣ�������������Ⱦ�������������ɣ����������������ʣ��ں������������Է��룬�ʴ�Ϊ��2LiCoO2+H2O2+3H2SO4
Li2SO4+2CoSO4+O2��+4H2O����������Ϣ֪LiCoO2����ǿ�����ԣ�������������Ⱦ�������������ɣ����������������ʣ��ں������������Է��룬�ʴ�Ϊ��2LiCoO2+H2O2+3H2SO4![]() Li2SO4+2CoSO4+O2��+4H2O�����������ɣ���Ⱦ�ϴ��������������ʣ��ں������������Է��룻
Li2SO4+2CoSO4+O2��+4H2O�����������ɣ���Ⱦ�ϴ��������������ʣ��ں������������Է��룻
��4���������������������ܺ�̼����立�Ӧ����̼���ܳ���������李�������̼��ˮ����Ӧ����ʽΪCoSO4+2NH4HCO3=CoCO3��+(NH4)2SO4+CO2��+H2O���ʴ�Ϊ��CoSO4+2NH4HCO3=CoCO3��+(NH4)2SO4+CO2��+H2O��
��5����ŵ�����У�Li1-xCoO2��LixC6����������ԭ��Ӧ����LiCoO2��C����Ӧ����ʽΪ��Li1-xCoO2+LixC6=LiCoO2+6C���ʴ�Ϊ��Li1-xCoO2+LixC6=LiCoO2+6C��

 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�