��Ŀ����
��֪��N2(g)+3H2(g) 2NH3(g)����H="-92.4" kJ/mol�����н�����ȷ����
2NH3(g)����H="-92.4" kJ/mol�����н�����ȷ����
| A�����ܱ������м���1 molN2(g)��3 molH2(g)��ַ�Ӧ����92.4 kJ |
B��N2(g)+3H2(g) 2NH3(l)����H="-Q" kJ/mol����Q��92.4 2NH3(l)����H="-Q" kJ/mol����Q��92.4 |
| C������ѹǿ��ƽ�������ƶ���ƽ�ⳣ������ |
| D����һ�������·�Ӧ�ﵽƽ�⣬N2��ת����Ϊ20%����H2��ת����һ��Ϊ60% |
B
�������������A�����ܱ������м���1 molN2(g)��3 molH2(g)�����ڷ�����N2(g)+3H2(g) 2NH3(g)��Ӧ�ǿ��淴Ӧ�����Գ�ַ�Ӧ����С��92.4 kJ ������B��N2(g)+3H2(g)
2NH3(g)��Ӧ�ǿ��淴Ӧ�����Գ�ַ�Ӧ����С��92.4 kJ ������B��N2(g)+3H2(g) 2NH3(l)����H="-Q" kJ/mol��������������̬ʱ���е�������Һ̬ʱ�ߣ����Ե�������Һ̬ʱ���ų�����������̬ʱҪ�ߣ������Q��92.4����ȷ��C������ѹǿ��ƽ�������ƶ�����������ƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ���Ͳ��䣬����D������Ӧ���ǰ���1:3���ʱ��һ�������·�Ӧ�ﵽƽ�⣬N2��ת����Ϊ20%����H2��ת����һ��Ϊ20%������
2NH3(l)����H="-Q" kJ/mol��������������̬ʱ���е�������Һ̬ʱ�ߣ����Ե�������Һ̬ʱ���ų�����������̬ʱҪ�ߣ������Q��92.4����ȷ��C������ѹǿ��ƽ�������ƶ�����������ƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ���Ͳ��䣬����D������Ӧ���ǰ���1:3���ʱ��һ�������·�Ӧ�ﵽƽ�⣬N2��ת����Ϊ20%����H2��ת����һ��Ϊ20%������
���㣺������������Ի�ѧƽ�⡢���ʵ�ת����ƽ�ⳣ����Ӱ���֪ʶ��

����������ʢ�й���ϡ������Թ��У���Ӱ�������������ʵ�������
| A�������Ũ�� | B�������ı���� | C����Һ���¶� | D��������Na2SO4���� |
�ڷ�Ӧ2SO2��O2 2SO3�У���a mol SO2��b mol O2�μӷ�Ӧ���ﵽ��ѧƽ��״̬ʱ��c mol SO3���ɣ���SO2��ƽ�������е��������Ϊ
2SO3�У���a mol SO2��b mol O2�μӷ�Ӧ���ﵽ��ѧƽ��״̬ʱ��c mol SO3���ɣ���SO2��ƽ�������е��������Ϊ
A��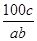 ��100% ��100% | B��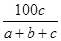 ��100% ��100% |
C��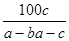 ��100% ��100% | D��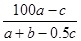 % % |
�����ͬ�ļס������������У��ֱ��������ʵ�����SO2��O2������ͬ�¶��·�����Ӧ��2SO2(g)+O2(g) 2SO3(g)�����ﵽƽ�⣬�ڴ˹����У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת����Ϊ
2SO3(g)�����ﵽƽ�⣬�ڴ˹����У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת����Ϊ
| A������p% | B������p% | C��С��p% | D�����Ƚ� |
����ƽ�ⳣ���У���ʾ������Ӧ�̶�������
A��K��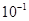 | B��K��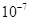 | C��K��1 | D��K��1010 |
 CH3OH(g) ��H��QkJ/mol
CH3OH(g) ��H��QkJ/mol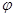 ( CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ2��ʾ����������Ӧ��Q_____0���������������������
( CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ2��ʾ����������Ӧ��Q_____0���������������������

 zC(g) ��H��0���ﵽ��ѧƽ�����A��Ũ��Ϊ0��20 mol��L-1�������¶Ȳ��䣬���ܱ��������ݻ���С��ԭ����һ�룬�ٴδﵽƽ��ʱ�����A��Ũ��Ϊ0��35 mol��L-1���������й��ж���ȷ����
zC(g) ��H��0���ﵽ��ѧƽ�����A��Ũ��Ϊ0��20 mol��L-1�������¶Ȳ��䣬���ܱ��������ݻ���С��ԭ����һ�룬�ٴδﵽƽ��ʱ�����A��Ũ��Ϊ0��35 mol��L-1���������й��ж���ȷ����