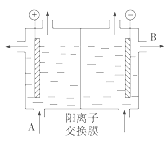
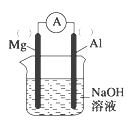
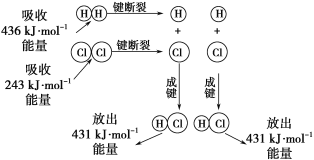
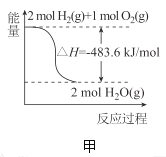
��Ŀ����
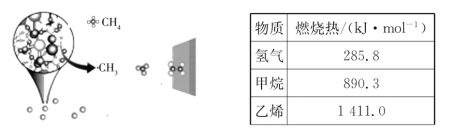
����Ŀ���п�Ժ������ѧ�����о�����һ�����³ɹ�ʵ���˼����Ч������ϩ����ͼ��ʾ�������ڴ����������⣬�ڲ�ͬ�¶��·ֱ��γ�![]() ��
��![]() ��
��![]() �����ɻ����������о����ɻ���CH2ż����Ӧ������ϩ(�÷�Ӧ���̿���)
�����ɻ����������о����ɻ���CH2ż����Ӧ������ϩ(�÷�Ӧ���̿���)
(1)��֪������ʵ�ȼ���������ʾ��д�������Ʊ���ϩ���Ȼ�ѧ����ʽ__________��
(2)�ִ�ʯ�ͻ�������Ag����������ʵ����ϩ�������Ʊ�X(����ʽΪC2H4O������˫��)�÷�Ӧ�����������ԭ�Ӿ��ã���Ӧ������__________(��ṹ��ʽ)
(3)��400��ʱ�����ʼ���Ϊ1L�ĺ�ѹ�ܱշ�Ӧ���г���1molCH4������(1)�з�Ӧ�����ƽ����������C2H4���������Ϊ25.0%����
���ڸ��¶��£���ƽ�ⳣ��KC��__________��
������÷�Ӧ����ͨ�����ˮ����(���μӷ�Ӧ������400��)����C2H4�IJ���__________��(�������С�������䡱����ȷ����)��������__________��
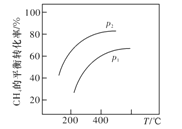
������Ӧ��������̶�����ͬѹǿ�¿ɵñ仯��ͼ��ʾ����ѹǿp1��p2�Ĵ�С��ϵ��__________��
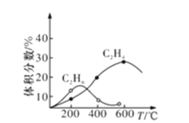
(4)ʵ���Ʊ�C2H4ʱ��ͨ�����ڸ���Ӧ2CH4(g)![]() C2H6(g)��H2(g)����Ӧ����CH4��ʼ�����䣬��ͬ�¶���C2H6��C2H4������������¶ȵĹ�ϵ������ͼ��ʾ�����¶ȸ���600��ʱ���п��ܵõ�һ�ֽ϶��˫̼�л��������������__________��
C2H6(g)��H2(g)����Ӧ����CH4��ʼ�����䣬��ͬ�¶���C2H6��C2H4������������¶ȵĹ�ϵ������ͼ��ʾ�����¶ȸ���600��ʱ���п��ܵõ�һ�ֽ϶��˫̼�л��������������__________��
(5)C2H4��C2H6������Ϊȼ�ϵ�ص�ԭ�ϣ���д��C2H4��NaOH��Һ����ȼ�ϵ�صĸ����ĵ缫��Ӧ����ʽ__________��
���𰸡�2CH4(g)C2H4(g)+2H2(g)��H=+202.0kJ/mol ![]() 1.0 ���� �÷�ӦΪ���������������ȷ�Ӧ��ͨ�����ˮ�����൱�ڼ��ȣ�ͬʱͨ��ˮ��������Ӧ������������൱�ڼ�Сѹǿ����ʹƽ�����ƣ�C2H4�IJ������� p1>p2 ��Ȳ 16OH��+C2H4��12e��=2
1.0 ���� �÷�ӦΪ���������������ȷ�Ӧ��ͨ�����ˮ�����൱�ڼ��ȣ�ͬʱͨ��ˮ��������Ӧ������������൱�ڼ�Сѹǿ����ʹƽ�����ƣ�C2H4�IJ������� p1>p2 ��Ȳ 16OH��+C2H4��12e��=2![]() +10H2O
+10H2O
��������
(1)���ݱ�����������дH2��CH4��C2H4ȼ���ȵ��Ȼ�ѧ����ʽ���ɸ�˹���ɼ��㣻
(2)X�ķ���ʽC2H4O������˫�����жϳ�X�Ľṹ��ʽ��
(3)�ٸ�������ʽ���ƽ����������C2H4���������Ϊ25.0%���㣻
��ͨ�����ˮ�������൱�ڼ��Ⱥͼ�Сѹǿ������(2)�еķ�Ӧ�����ƽ���Ӱ�����ط������
������������̶������ݷ�Ӧ���������ѹǿ��ƽ���Ӱ������жϣ�
(4)��ͼ��֪���¶ȸ���600��ʱ���н϶�����ɻ�![]() ���ɣ�
���ɣ�
(5)ȼ�ϵ���У�ͨ��ȼ�ϵ�һ��Ϊ������ͨ��������һ��Ϊ�������ݴ˷�����д�缫��Ӧ��
(1)���ݱ����������У���H2(g)+![]() O2(g)�TH2O(l)��H1=-285.8kJ/mol��
O2(g)�TH2O(l)��H1=-285.8kJ/mol��
��CH4(g)+2O2(g)=CO2(g)+2H2O(l)��H2=-890.3kJ/mol��
��C2H4(g)+3O2(g)=2CO2(g)+2H2O(l)��H3=-1411.0kJ/mol��
�����Ʊ���ϩ�Ļ�ѧ����ʽΪ��2CH4(g)C2H4(g)+2H2(g)�����ݸ�˹���ɣ�������2-��-����2�õ���2CH4(g)C2H4(g)+2H2(g)��H=2��H2-��H3-2��H1=+202.0kJ/mol��
(2)��������ϩ���������Ʊ�X��X�ķ���ʽC2H4O������˫������Ӧ�����������ԭ�Ӿ��ÿ�֪��X�Ľṹ��ʽΪ![]() ��
��
(3)��400��ʱ����1L�ĺ��ݷ�Ӧ���г���1molCH4������������Ӧ�����ƽ����������C2H4���������Ϊ25.0%����ת���ļ���Ϊx���ɴ˽�����������ʽ��
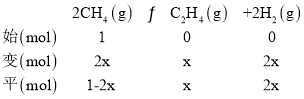
��ͬ���������������ȵ������ʵ���֮�ȣ�������![]() =25%�����x=
=25%�����x=![]() mol��ƽ��ʱ����������ʵ���Ϊ(
mol��ƽ��ʱ����������ʵ���Ϊ(![]() +
+![]() +
+![]() )mol=
)mol=![]() mol��������ѹ������������ʵ���֮�ȵ������֮�ȣ�ԭ���Ϊ1L�����ʱ���ӦΪ
mol��������ѹ������������ʵ���֮�ȵ������֮�ȣ�ԭ���Ϊ1L�����ʱ���ӦΪ![]() L�����Ի�ѧƽ�ⳣ��KC=
L�����Ի�ѧƽ�ⳣ��KC=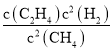 =
= =1.0��
=1.0��
�ڼ����Ʊ���ϩ�ķ�ӦΪ���������������ȷ�Ӧ��ͨ�����ˮ�������൱�ڼ��ȣ�ƽ�����ƣ���������ͬʱͨ��ˮ��������������������൱�ڼ�Сѹǿ��ƽ�����ƣ�����Ҳ�������C2H4�IJ��ʽ�����
������������̶��������Ʊ���ϩ�ķ�ӦΪ�����������ķ�Ӧ���¶���ͬʱ������ѹǿƽ�����淴Ӧ�����ƶ���CH4��ƽ��ת���ʽ��ͣ����p1��p2��
(4)��ͼ��֪�¶ȸ���600��ʱ��Ӧ�н϶�����ɻ�![]() ���ɣ����ɻ��������
���ɣ����ɻ��������![]() ����˫̼�л�������Ϊ��Ȳ��
����˫̼�л�������Ϊ��Ȳ��
(5)ȼ�ϵ��ͨ��ȼ�ϵ�һ��Ϊ�������������ϼ����ߣ�C2H4��NaOH��Һ��ת��ΪCO32-���缫��ӦʽΪ��C2H4-12e-+16OH-=2CO32-+10H2O��

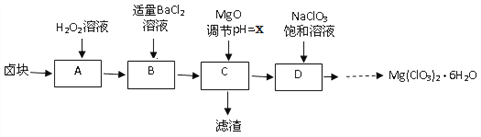
����Ŀ��ʵ������±�飨��Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4.FeCl2�����ʣ��Ʊ�����Mg(ClO3)2��6H2O���������£�

��֪�������ֻ�������ܽ��(S)���¶�(T)�仯������ͼ��ʾ��
������ʱһЩ���ʵ�Ksp���±���
��ѧʽ | Fe(OH)2 | Fe(OH)3 | Mg(OH)2 |
Ksp | 8.0��10-16 | 8.0��10-38 | 1.8x10-11 |
��Mg(ClO3)2�н�ǿ�������ԣ��仹ԭ������Cl-.
��1��H2O2�ĵ���ʽΪ_________
��2�������ijɷ���____________���ѧʽ����
��3�����ⶨ��D�������ӵ�Ũ��Ϊ1��10-5 mol/L,��xΪ______
��4��D���������Ļ�ѧ��Ӧ����ʽΪ_____����ͼ����D��......����Mg(ClO3)2��6H2O�����ʵ�鲽������Ϊ���ټ�����������_______���벹�䣩������ȴ�ᾧ���ܹ���ϴ�ӡ�
��5����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10.00 mL��Һ����ƿ�У�����10.00 mLϡ�����20 .00mL 1.000 mol/L��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol/L K2Cr2O7��Һ�ζ�ʣ���Fe2�����յ㡣
����4��������2��3�ظ�����
�ٲ���3�з�����Ӧ�����ӷ���ʽ____________
�ڲ���3�����ζ�ǰ���ñ�Һ��ϴ�ζ��ܣ����ᵼ�����ս��_____������ƫ����. ��ƫС����������������
����ƽ������K2Cr2O7��Һ15.00 mL�����Ʒ��Mg(ClO3)2��6H2O����M=299g/mol������������Ϊ___________
����Ŀ����֪��ӦA(g)��B(g) ![]() C(g)��D(g)��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ�����ʾ��830 ��ʱ����һ��2 L���ܱ������г���0.20 mol A��0.20 mol B,10 sʱ��ƽ�⡣����˵������ȷ����
C(g)��D(g)��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ�����ʾ��830 ��ʱ����һ��2 L���ܱ������г���0.20 mol A��0.20 mol B,10 sʱ��ƽ�⡣����˵������ȷ����
�¶�/�� | 700 | 830 | 1200 |
Kֵ | 1.7 | 1.0 | 0.4 |
A. �ﵽƽ���B��ת����Ϊ50%
B. ����ѹǿ�������淴Ӧ���ʾ��ӿ�
C. �÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�
D. ��Ӧ��ʼ��ƽ�⣬A��ƽ����Ӧ����v(A)��0.005 mol��L��1��s��1