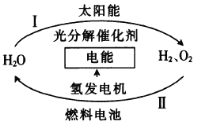
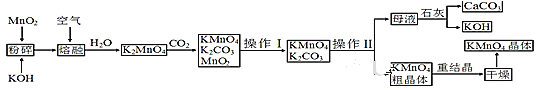
��Ŀ����
����Ŀ����25�桢101kPa�������£�����1molH��H������436kJ����������1molCl��Cl������243kJ�������γ�1molH-Cl���ų�431 kJ������H2+Cl2=2HCl�Ļ�ѧ��Ӧ������ͼ��ʾ��
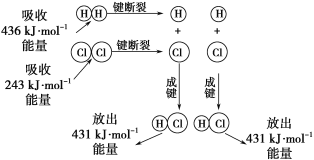
��ش������й����⣺
(1)��Ӧ��ϼ����յ�������Ϊ___________________��
(2)������ɼ��ų���������Ϊ______________��
(3)�ж�H2+Cl2=2HCl��Ӧ_____________(���������������ų���)������
(4)��Ӧ���������________(������������=����������)���������������
���𰸡�679kJ 862kJ �ų� >
��������
(1)��Ӧ��ϼ����յ�������Ϊ�����������ļ��ܺͣ�
(2)������ɼ��ų���������Ϊ2molHCl���ܼ��ܣ�
(3)����������ļ��ܵĺ��뷴Ӧ��ļ��ܵĺ͵Ĵ�С��ϵ�жϡ������Ƚϡ�
(1)��Ӧ��ϼ����յ�������Ϊ�����������ļ��ܺͣ���Ӧ��ϼ����յ�������Ϊ436kJ+243kJ=679kJ��
(2)������ɼ��ų���������Ϊ�γ�2molHCl���ܼ��ܣ���������ɼ��ų���������Ϊ431kJ��2=862kJ��
(3)�ϼ����յ���������679kJ���γ�2molHCl�ͷŵ���������862kJ�����յ������٣��ų��������࣬��˸÷�Ӧ�Ƿų������ķ�Ӧ��
(4)��Ӧ�ų�������˵����Ӧ����������������������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�