��Ŀ����
5�������йػ�ѧ��Ӧ������ȷ���ǣ�������| A�� | ���ð�˾ƥ�ֹ�������ˮ���ᣨ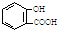 ���ж���Ӧ���ɾ���ע��NaHCO3��Һ�� ���ж���Ӧ���ɾ���ע��NaHCO3��Һ��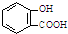 +2HCO3-�� +2HCO3-��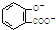 +2 CO2��+2 H2O +2 CO2��+2 H2O | |
| B�� | ��ȩ��Һ��������������Һ���ȣ�HCHO+4[Ag��NH3��2]++4OH-$\stackrel{��}{��}$CO32-+2NH4++4Ag��+6NH3+2H2O | |
| C�� | ��CH2BrCOOH�м�������������������Һ�����ȣ�CH2BrCOOH+OH-$\stackrel{��}{��}$CH2BrCOO-+H2O | |
| D�� | ��������Һ��ͨ������CO2��CO2+H2O+2C6H5O-��2C6H5OH+2CO32- |
���� A�����ǻ���̼�����Ʋ���Ӧ��
B������������Ӧ����̼��李�����������ˮ��
C��-Br��-COOH����NaOH��Ӧ��
D����������Һ��ͨ������CO2��Ӧ���ɱ��Ӻ�̼�����ƣ�
��� �⣺A�����ð�˾ƥ�ֹ�������ˮ���ᣨ ���ж���Ӧ���ɾ���ע��NaHCO3��Һ�����ӷ���ʽΪ��
���ж���Ӧ���ɾ���ע��NaHCO3��Һ�����ӷ���ʽΪ�� +HCO3-��
+HCO3-�� +2CO2��+H2O����A����
+2CO2��+H2O����A����
B��ȩ��Һ��������������Һ���ȵ����ӷ�ӦΪHCHO+4[Ag��NH3��2]++4OH-$\stackrel{��}{��}$CO32-+2NH4++4Ag��+6NH3+2H2O����B��ȷ��
C����CH2BrCOOH�м�������������������Һ�����ȵ����ӷ�ӦΪCH2BrCOOH+2OH-$\stackrel{��}{��}$CH2OHCOO-+Br-+H2O����C����
D����������Һ��ͨ������CO2�����ӷ�ӦΪC6H5O-+CO2+H2O��C6H5OH+HCO3-����D����
��ѡB��
���� ���⿼�����ӷ�Ӧ����ʽ��д�������жϣ�Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ������������йص����ӷ�Ӧ�����ֽⷴӦ�����ӷ�Ӧ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
11���������ʲ����ڵ���ʣ�����ˮ��Һ�ܵ�����ǣ�������
| A�� | HCl | B�� | BaCO3 | C�� | NaCl | D�� | SO3 |
20������ʵ�����������ͽ��۾���ȷ���ǣ�������
| ѡ�� | ʵ����� | ���� | ���� |
| A | պ��Ũ��ˮ�IJ���������ij��Һ | �а��̲��� | ����Һ������Ũ���� |
| B | ��SO2ͨ��Ba��NO3��2��Һ | ������ɫ���� | SO2������Ա��ξ������ɰ�ɫ���� |
| C | ����������Һ�еμӹ���������������Һ | ������ɫ���� | �������������ڹ���������������Һ |
| D | �ò�˿պȡ����ij��Һ������ɫ��Ӧ | ����ʻ�ɫ | ����Һһ����������Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
14�����е���ʽ����ȷ���ǣ�������
����ԭ��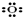 �ڹ���������
�ڹ��������� ������������
������������ ��������[Na]+
��������[Na]+
��������H+��笠�����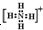 ����ԭ��
����ԭ�� ���帺����
���帺����
����ԭ��
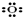 �ڹ���������
�ڹ��������� ������������
������������ ��������[Na]+
��������[Na]+��������H+��笠�����
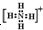 ����ԭ��
����ԭ�� ���帺����
���帺����
| A�� | �٢ڢۢޢ� | B�� | �ۢܢޢߢ� | C�� | �ۢݢޢߢ� | D�� | �ڢۢܢߢ� |
15�����ʵ�顰�����������롰���ۡ���Ӧ��ϵ��������ǣ�������
| ѡ�� | ���������� | ���� |
| A | ��װ��Fe��NO3��2��Һ���Թ��м���ϡH2SO4�����Թܿڹ۲쵽����ɫ���� | ������Ӧ������NO��������O2��Ӧ����NO2 |
| B | ȡ������Ӧ�Ļ��Һ����ʢˮ���ձ��У������͵� | ������Ӧ��ȫ |
| C | ���������м���NaOH���Ҵ���Һ�����ȣ�����������������ͨ��ˮ������KMnO4��Һ��KMnO4��Һ�Ϻ�ɫ��ɫ | ʹKMnO4��Һ��ɫ����������ϩ |
| D | ��Ư����Һ��ͨ������Ķ���������Һ����� | ����������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��
��
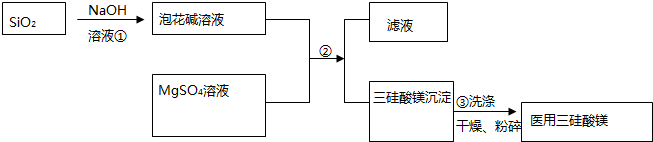

 ��
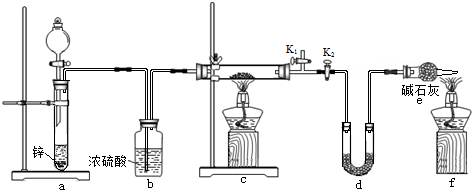
�� ��˹����APM����һ����ȸߡ�ζ���������͵���ζ������ṹ��ʽ��ͼ��ʾ��
��˹����APM����һ����ȸߡ�ζ���������͵���ζ������ṹ��ʽ��ͼ��ʾ��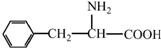 �Ǻϳ�APM��ԭ��֮һ�����������һ�ֺϳ�;����ͼ��ʾ��
�Ǻϳ�APM��ԭ��֮һ�����������һ�ֺϳ�;����ͼ��ʾ��
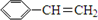 ��
��
 ��
��