��Ŀ����
14��CO2����ˮ����̼�ᣮ��֪�������ݣ�| ������� | H2CO3 | NH3•H2O |
| ����ƽ�ⳣ����25�棩 | ${K}_{{a}_{1}}$=4.30��107 ${K}_{{a}_{2}}$=5.61��10-11 | Kh=1.77��10-5 |
����Kh=$\frac{{K}_{w}}{{K}_{{a}_{2}}}$������˵����ȷ���ǣ�
| A�� | �����ݿ��жϸ���Һ������ | |
| B�� | c��NH4+����c��HCO32-����c��CO32-����c��NH3•H2O�� | |
| C�� | c��NH4+��+c��NH3•H2O��=2c��CO32-��+2c��HCO3-��+2c��H2CO3�� | |
| D�� | c��NH4+��+c��H+��=c��HCO3-��+c��OH-��+c��CO32-�� |
���� A������CO32-��һ��ˮ���ƽ�ⳣ������NH4+ˮ���ƽ�ⳣ����֪��Һ�ʼ��ԣ�
B��̼�������ˮ��̶Ƚ�С����c��CO32-����c��HCO3-����
C������̼�����Һ�е������غ��жϣ�
D������̼�����Һ�еĵ���غ��жϣ�
��� �⣺A��CO32-��һ��ˮ���ƽ�ⳣ��$\frac{{K}_{W}}{5.61��1{0}^{-11}}$����NH4+ˮ���ƽ�ⳣ��$\frac{{K}_{W}}{1.77��1{0}^{-5}}$������Һ�ʼ��ԣ���A����
B��笠����ӵ�ˮ��̶���Ҫ̼������ӣ�����Һ��c��NH4+����c��CO32-����c��HCO3-����c��NH3•H2O��������ˮ��̶Ƚ�С����c��CO32-����c��HCO3-����������Һ������Ũ�ȴ�СΪ��c��NH4+����c��CO32-����c��HCO3-����c��NH3•H2O������B����
C��������Һ�������غ�õ���c��NH4+��+c��NH3•H2O��=2c��CO32-��+2c��HCO3-��+2c��H2CO3��=2mol•L-1 ����C��ȷ��
D����Һ�д��ڵ���غ�Ϊ��c��NH4+��+c��H+��=c��HCO3-��+c��OH-��+2c��CO32-������D����
��ѡC��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ̼������ӡ�笠����ӵ�ˮ��̶ȴ�СΪ���ؼ���ע�����յ���غ㡢�����غ㼰�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�÷�����

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�| A�� | ������������ˮ���ʻ��о����������ζ | |
| B�� | ����ͼ״���һ��������Ҳ�ܷ���������Ӧ | |
| C�� | ������ӦҲ����ȡ����Ӧ | |
| D�� | ������Ӧ����Ҫϡ���������� |
| A�� | �¶�Խ�ߣ������Ĵ�Ч��Խ�� | |
| B�� | �����������ϵġ���ת�������ܼ����к������ŷ� | |
| C�� | �������Ըı仯ѧ��Ӧ���ʣ��������ƻ���ѧƽ�� | |
| D�� | �������Ըı仯ѧ��Ӧ·�����ҷ�Ӧǰ���������ͻ�ѧ���ʶ��������仯 |
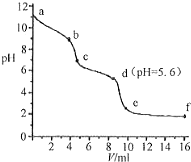 �����£�����֪Ũ�ȵ�����ζ���δ֪Ũ�ȵ�Na2CO3��Һ����pH��������û����Һ��pH�仯���ߣ���ͼ��ʾ������֪����CO2��Һ��pHΪ5.6������˵����ȷ���ǣ�������
�����£�����֪Ũ�ȵ�����ζ���δ֪Ũ�ȵ�Na2CO3��Һ����pH��������û����Һ��pH�仯���ߣ���ͼ��ʾ������֪����CO2��Һ��pHΪ5.6������˵����ȷ���ǣ�������| A�� | a��ʱ����Һ�ʼ��Ե�ԭ����CO32-����ˮ�ⷴӦ�������ӷ���ʽΪ��CO32-+2H2O=H2CO3+2OH- | |
| B�� | a��b�Σ���Һ������ų� | |
| C�� | c���Ժ�������� | |
| D�� | d����Һ��c��Na+��=c��Cl-�� |
| A�� | ����ͨ�����˵ķ��������۽����л��е��Ȼ�����Һ��ȥ | |
| B�� | ��2mL10%��CuSO4��aq���е���3�� 2%��NaOH��aq�������Ƽ���ȩ�����Լ� | |
| C�� | ��������ƽȷ��ȡ14.80g Ca��OH��2������100mL 2.0 mol/L��Ca��OH��2��Һ | |
| D�� | ���������������Ҵ����ʿɼ�ˮ��ȥ����ԭ������ˮ�м����Ȼ�̼��ȡ������ |
 ��1.00mol•L-1 NaOH��Һ����20.00mL 1.00mol•L-1��һԪ��HA��Һ�У���û����Һ��pH���¶������NaOH��Һ����仯������ͼ��ʾ������˵����ȷ���ǣ�������
��1.00mol•L-1 NaOH��Һ����20.00mL 1.00mol•L-1��һԪ��HA��Һ�У���û����Һ��pH���¶������NaOH��Һ����仯������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ����ʱ��HA�ĵ����ԼΪ10-6 | |
| B�� | ͼ��B��ʱ����Һ��KW�Դ���1��10-14 | |
| C�� | ͼ��C��ʱ����Һ��c��A-��=c��Na+����c��H+��=c��OH-�� | |
| D�� | ͼ��D�����Һ�¶����½�����Ҫԭ�������ɵ� NaAˮ������ |
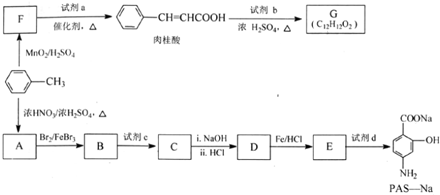
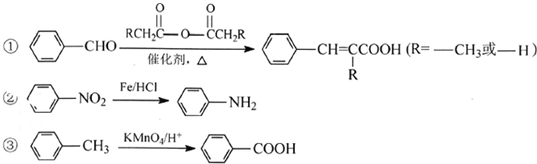
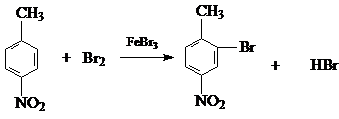 ��
��  ��
��  ��
�� ��
��