��Ŀ����
����Ŀ��ԭ�����������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ�����AԪ��ԭ�Ӻ���һ�����ӣ�BԪ��ԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��B��C���γ����������η��ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����ش��������⣺
��1��������Ԫ���е縺��������________(��Ԫ�ط���)����һ��������С����________(��Ԫ�ط���)��
��2��C���ڵ�����Ԫ����̬�⻯���У��е���͵���________(�ѧʽ)��
��3��BԪ�ؿ��γɶ��ֵ��ʣ����С�ֻ��һ��ԭ�Ӻ����ʣ�������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ��ò��Ͼ���ṹ��ͼ��ʾ����ԭ�ӵ��ӻ�����Ϊ________�ӻ���

��4��D��ˮ�ϴ����ξ���ֲ��ṹ��ͼ��ʾ���þ����к��еĻ�ѧ����________(��ѡ�����)��

�ټ��Լ����ڷǼ��Լ�������λ�����ܽ�����
���𰸡�Cl Cu HCl sp2 �٢ڢ�
��������
ԭ�����������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ�����Aԭ�Ӻ���һ�����ӣ���AΪHԪ�أ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬����������6�����ӣ���BΪCԪ�أ�B��C���γ����������ͷ��ӣ���C���ڵ������ڣ���CΪClԪ�أ�Dԭ����Χ�����Ų�Ϊ3d104s1����DΪCuԪ�أ�
��1������Ԫ���У���Ԫ�صķǽ�������ǿ����縺�����ͭΪ����������Ϊ�ǽ���������ͭ��һ��������С���ʴ�Ϊ��Cl��Cu��
��2��CΪ��A��Ԫ�أ�HF�д���������е��HCl�ߣ������⻯����Է�������Խ�е�Խ�ߣ�����HCl�ķе���ͣ��ʴ�Ϊ��HCl��
��3�������״�ṹ��̼̼������Ϊ120�㣬ÿ��̼ԭ�Ӷ������3��̼ԭ�ӣ�̼ԭ�Ӽ��γ������м����ʴ�Ϊ��sp2��
��4���ɽṹͼ��֪���þ����к���C-H��Ϊ���Լ���C-C��Ϊ�Ǽ��Լ�����λ������ѡ���٢ڢ���

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
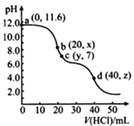
��У������Ԫͬ��ѵ��������ϵ�д�����Ŀ��KIO3 ��һ����Ҫ�������������Ϊʳ���еIJ������������KClO3 ���������Ʊ� KIO3 ������������ͼ��ʾ���ش��������⣺
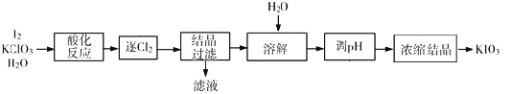
(1)���ữ��Ӧ�����ò����� KH(IO3)2��Cl2 �� KCl������ Cl2�����õķ�����________��
(2)����Һ���е�������Ҫ��_______������ pH���з�����Ӧ�Ļ�ѧ����ʽΪ_____________________��
(3)�ᾧ����ʱ���˷��� KH(IO3)2 Ϊ������״���������¿��Բ��õķ�����________��
A�������� B.�ؽᾧ�� C.���˷� D. ���ķ��뷨
(4)�����У����ʡȥ���ữ�����������������ᾧ�١�������������������ֱ�����Լ� X ������Ӧ����Һ�� pH���������������ʲô����Ӱ��_________________________________________________________________��
(5)KIO3 Ҳ�ɲ�������ⷨ���Ʊ���װ����ͼ��ʾ��������ⷨ����ȣ���KClO3 ������������Ҫ����֮����_____________________________________��(д��һ��)

(6)��֪��KIO3��5KI��3H2SO4=3K2SO4��3I2��3H2O��I2��2S2O32-=2I����S4O62-���ⶨ�ӵ�ʳ���е�ĺ�����ѧ������Ƶ�ʵ�鲽�����£�
a��ȷ��ȡ w g ʳ�Σ�����������ˮʹ����ȫ�ܽ⣻
b����ϡ�����ữ������Һ��������� KI ��Һ��ʹ KIO3 �� KI ��Ӧ��ȫ��
c���Ե���Ϊָʾ�����������ʵ���Ũ��Ϊ2.0��10-3mol��L-1 �� Na2S2O3 ��Һ 10.0mL ǡ�÷�Ӧ�� ��ӵ�ʳ����Ʒ�еĵ�Ԫ�غ�����______________mg/kg��(�Ժ� w �Ĵ���ʽ��ʾ)
(7)ѧ�����ֶԴ����� NaCl(���� KIO3)����������ʵ�飺
�������� | ʵ������ |
ȡ 1g ������ NaCl���� 3mL ˮ�����Һ�� | ��Һ�ޱ仯 |
���� 5 �ε�����Һ�� 1mL 0.1 mol��L��1KI ��Һ���� | ��Һ�ޱ仯 |
Ȼ���ٵ��� 1 �� 1mol��L��1 �� H2SO4���� | ��Һ����ɫ |
����ѧ���ҵ�ʵ���������ѧ����ʵ����������Ҫ����___________________________��