��Ŀ����
����Ŀ���������������ʵ���Һ��
��NaCl ��NH4Cl ��Na2CO3 ��CH3COONa ��CH3COOH ��NaHCO3
(1)��Һ�����Ե���_____________���ʼ��Ե���____________�������Ե���_____________��
(2)д���ڢ�ˮ������ӷ���ʽ��________________________��______________________
��3�������£�Ũ�Ⱦ�Ϊ0.1mol/L������������Һ����������_____________(����ͬ���Dz���ͬ������Һ��PH:��_________��(�� >������ <)��
(4)������0.1 mol/L��CH3COOH��Һ��ˮϡ�����У����б���ʽ������һ��������______________��
A��c(H��) B.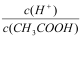 C��c(H��)��c(OH��)
C��c(H��)��c(OH��)
���𰸡�(1)�ڢ� �ۢܢ� ��
(2)NH![]() ��H2O
��H2O![]() NH3��H2O��H��
NH3��H2O��H��
CH3COO����H2O![]() CH3COOH��OH��
CH3COOH��OH��
��3����ͬ > ��4��B
��������
�����������1����NaCl ����ǿ��ǿ��������Һ�����ԣ���NH4Cl ��ǿ�������Σ���Һ��笠�����ˮ������Һ�����ԣ���Na2CO3��ǿ�������Σ���Һ��̼�������ˮ����Һ�Լ��ԣ���CH3COONa ��ǿ�������Σ���Һ�д��������ˮ����Һ�Լ��ԣ���NaHCO3 ��ǿ�������Σ���Һ��̼���������ˮ����Һ�Լ��ԡ�����������֪��Һ�����Ե����ڢ�����Һ�ʼ��Ե����ۢܢ��������Ե�������
��2����NH4Cl ��ǿ�������Σ���Һ��笠�����ˮ������Һ�����ԣ�ˮ�����ӷ���ʽΪNH![]() ��H2O
��H2O![]() NH3��H2O��H������CH3COONa ��ǿ�������Σ���Һ�д��������ˮ�⣬��Һ�Լ�����ˮ�����ӷ���ʽΪCH3COO����H2O
NH3��H2O��H������CH3COONa ��ǿ�������Σ���Һ�д��������ˮ�⣬��Һ�Լ�����ˮ�����ӷ���ʽΪCH3COO����H2O![]() CH3COOH��OH����
CH3COOH��OH����
��3��̼������Һ�д���ˮ�ĵ����CO32-��ˮ����̣�ˮ����������У���Һ�е�������H+��OH����CO32-��HCO3-��Na����̼��������Һ�д���ˮ�ĵ��롢HCO3-��ˮ��ƽ��͵���ƽ�⣬��Һ�е�������H+��OH����CO32-��HCO3-��Na�������Գ����£�Ũ�Ⱦ�Ϊ0.1mol/L������������Һ������������ͬ������H2CO3>HCO3-������Խ��Խˮ�⣬Ũ����ͬʱ��̼������ӵ�ˮ��̶ȴ���̼��������ӵ�ˮ��̶ȣ�ˮ��̶�Խ����Һ�ļ���Խǿ��pHԽ������Һ��PH: ��>����
��4�������£�0.1 mol/L��CH3COOH��Һ��ˮϡ�����У�����ĵ���̶��������Լ�����c(H��)��С����Һ��n(H��)���࣬n(CH3COOH)��С��![]() �����¶Ȳ��䣬c(H��)��c(OH��)=Kw���䣬��ѡB��
�����¶Ȳ��䣬c(H��)��c(OH��)=Kw���䣬��ѡB��

 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� |
(1)����ЩԪ���У���ѧ�������������:_____(��Ԫ�ط���)��ԭ�ӽṹʾ��ͼΪ_____ ��
(2)������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ��_______��������ǿ�Ļ����������ʽ��:_____________��
(3)�õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣�________���û���������_____(�� �����ۡ������ӡ�)�����
(4)��ʾ����H�Ļ�����Ļ�ѧʽ_________________���û���������____________������ԡ����Ǽ��ԡ������γɵġ�
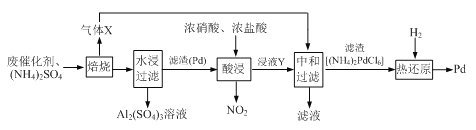
����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ��
����Cr(��)�Ĵ��������������£�

��֪���������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
������ȫʱ��pH | 3.7 | 11.1 | 5.4(>8�ܽ�) | 9(>9�ܽ�) |
(1)ʵ������18.4 mol��L-1��Ũ��������480 mL 2 mol��L��1�����ᣬ����ȡŨ����___mL������ʱ���ò�����������Ͳ���ձ��Ͳ������⣬����____________________________��
(2)H2O2�������ǽ���Һ���е�Cr3+ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ��
___________________________________________��
(3)����NaOH��Һʹ��Һ�ʼ��ԣ��ȿ��Գ�ȥijЩ�������ӣ�ͬʱ�ֿ��Խ�Cr2O72-ת��Ϊ__________(�����Ļ�ѧʽ)
(4)�����ӽ�����֬�ķ�Ӧԭ��Ϊ��Mn+ + n NaR = MRn + n Na���������������ӽ�����֬�ɳ�ȥ��Һ���еĽ�����������__________________��
(5)д��������������SO2���л�ԭʱ������Ӧ�����ӷ���ʽ______________________________��
(6)�����ζ����Dzⶨ����Ũ�ȵķ���֮һ��Ϊ�˲ⶨij��ˮ��SCN��Ũ�ȣ����ñ�AgNO3��Һ�ζ�����Һ����֪��
�������� | AgCl | AgI | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | �� | �� | ש�� | �� |
Ksp | 1.8��10-10 | 8.3��10-17 | 1.2��10-16 | 3.5��10-11 | 1.0��10-12 |
�ζ�ʱ��ѡΪ�ζ�ָʾ������____(ѡ����)���ζ��յ�������________________________��
A��NaCl B��K2CrO4 C��KI D��NaCN