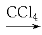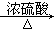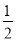ЬтФПФкШн
ЫцзХЪРНчЙЄвЕОМУЕФЗЂеЙЁЂШЫПкЕФОчдіЃЌШЋЧђФмдДНєеХМАЪРНчЦјКђУцСйдНРДдНбЯжиЕФЮЪЬтЃЌШчКЮНЕЕЭДѓЦјжаCO2ЕФКЌСПМАгааЇЕиПЊЗЂРћгУCO2в§Ц№СЫШЋЪРНчЕФЦеБщжиЪгЁЃ
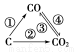
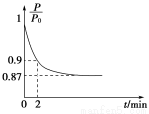
ЃЈ1ЃЉШчЭМЮЊCМАЦфбѕЛЏЮяЕФБфЛЏЙиЯЕЭМЃЌШєЂйБфЛЏЪЧжУЛЛЗДгІЃЌдђЦфЛЏбЇЗНГЬЪНПЩЮЊ______________________________________ЃЛ
ЭМжаБфЛЏЙ§ГЬФФаЉЪЧЮќШШЗДгІ________ЃЈЬюађКХЃЉЁЃ
ЃЈ2ЃЉМзДМЪЧвЛжжПЩдйЩњФмдДЃЌОпгаПЊЗЂКЭгІгУЕФЙуРЋЧАОАЃЌЙЄвЕЩЯПЩгУШчЯТЗНЗЈКЯГЩМзДМЃК
ЗНЗЈвЛЁЁCOЃЈgЃЉЃЋ2H2ЃЈgЃЉ??CH3OHЃЈgЃЉ
ЗНЗЈЖўЁЁCO2ЃЈgЃЉЃЋ3H2ЃЈgЃЉ??CH3OHЃЈgЃЉЃЋH2OЃЈgЃЉ
дк25ЁцЁЂ101 kPaЯТЃЌ1ПЫМзДМЭъШЋШМЩеЗХШШ22.68 kJЃЌаДГіМзДМШМЩеЕФШШЛЏбЇЗНГЬЪНЃК_____________________________________________ЃЛ
ФГЛ№СІЗЂЕчГЇCO2ЕФФъЖШХХЗХСПЪЧ2 200ЭђЖжЃЌШєНЋДЫCO2ЭъШЋзЊЛЏЮЊМзДМЃЌдђРэТлЩЯгЩДЫЛёЕУЕФМзДМЭъШЋШМЩеЗХШШдМЪЧ________kJЃЈБЃСєШ§ЮЛгааЇЪ§зжЃЉЁЃ
ЃЈ3ЃЉН№ЪєюбвБСЖЙ§ГЬжаЦфжавЛВНЗДгІЪЧНЋдСЯН№КьЪЏзЊЛЏЃКTiO2ЃЈН№КьЪЏЃЉЃЋ2CЃЋ2Cl2ИпЮТ,TiCl4ЃЋ2COЁЁвбжЊЃКCЃЈsЃЉЃЋO2ЃЈgЃЉ=CO2ЃЈgЃЉЁЁІЄHЃНЃ393.5 kJЁЄmolЃ1
2COЃЈgЃЉЃЋO2ЃЈgЃЉ=2CO2ЃЈgЃЉЁЁІЄHЃНЃ566 kJЁЄmolЃ1
TiO2ЃЈsЃЉЃЋ2Cl2ЃЈgЃЉ=TiCl4ЃЈsЃЉЃЋO2ЃЈgЃЉЁЁІЄHЃНЃЋ141 kJЁЄmolЃ1
дђTiO2ЃЈsЃЉЃЋ2Cl2ЃЈgЃЉЃЋ2CЃЈsЃЉ=TiCl4ЃЈsЃЉЃЋ2COЃЈgЃЉЕФІЄHЃН________ЁЃ
ЃЈ4ЃЉГєбѕПЩгУгкОЛЛЏПеЦјЃЌвћгУЫЎЯћЖОЃЌДІРэЙЄвЕЗЯЮяКЭзїЮЊЦЏАзМСЁЃГєбѕМИКѕПЩгыçЁЂН№ЁЂвПЁЂЗњвдЭтЕФЫљгаЕЅжЪЗДгІЁЃШчЃК
6AgЃЈsЃЉЃЋO3ЃЈgЃЉ=3Ag2OЃЈsЃЉЁЁІЄHЃНЃ235.8 kJЁЄmolЃ1ЃЌ
вбжЊЃК2Ag2OЃЈsЃЉ=4AgЃЈsЃЉЃЋO2ЃЈgЃЉІЄHЃНЃЋ62.2 kJЁЄmolЃ1ЃЌ
дђO3зЊЛЏЮЊO2ЕФШШЛЏбЇЗНГЬЪНЮЊ_________________________ЁЃ
ЃЈ1ЃЉCЃЋH2OЃЈgЃЉИпЮТ,H2ЃЋCOЃЈКЯРэМДПЩЃЌШчгыCuOЁЂFeOЁЂSiO2ЕШЗДгІЃЉЁЁЂйЂл
ЃЈ2ЃЉCH3OHЃЈlЃЉЃЋ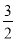 O2ЃЈgЃЉ=CO2ЃЈgЃЉЃЋ2H2OЃЈlЃЉЁЁІЄHЃНЃ725.76 kJЁЄmolЃ1ЁЁ3.63ЁС1014
O2ЃЈgЃЉ=CO2ЃЈgЃЉЃЋ2H2OЃЈlЃЉЁЁІЄHЃНЃ725.76 kJЁЄmolЃ1ЁЁ3.63ЁС1014
ЃЈ3ЃЉЃ80 kJЁЄmolЃ1
ЃЈ4ЃЉ2O3ЃЈgЃЉ=3O2ЃЈgЃЉЁЁІЄHЃНЃ285 kJЁЄmolЃ1
ЁОНтЮіЁПЃЈ1ЃЉгЩаХЯЂПЩжЊЗДгІЂйЪЧжУЛЛЗДгІЃЌЫљвдгІЮЊЮќШШЗДгІЃЛЃЈ2ЃЉ1 molМзДМШМЩеЗХГіШШСП22.68 kJЁС32ЃН725.76 kJЃЌвРОнаХЯЂЩњГЩЮяЫЎЮЊвКЬЌЃЛвРОнЬМдзгЪиКуnЃЈCH3OHЃЉЃНnЃЈCO2ЃЉЃН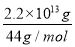 ЃН5ЁС1011 molЃЌЗХГіШШСП725.76 kJЁЄmolЃ1ЁС5ЁС1011 molЃН3.63ЁС1014 kJЃЛЃЈ3ЃЉЖдвбжЊШШЛЏбЇЗНГЬЪНБрКХДгЧАЕНКѓЗжБ№ЮЊЂйЂкЂлЃЌЂлЃЂкЃЋЂйЁС2МДПЩЕУЫљЧѓЗНГЬЪНЃЌЫљвдІЄHЃНЃЋ141 kJЁЄmolЃ1ЃЃЈЃ566 kJЁЄmolЃ1ЃЉЃЋЃЈЃ393.5 kJЁЄmolЃ1ЃЉЁС2ЃЛЃЈ4ЃЉЪзЯШаДГіЫљЧѓЗДгІЕФЛЏбЇЗНГЬЪН2O3ЃЈgЃЉ=3O2ЃЈgЃЉЃЌЧАЪНЁС2ЃЋКѓЪНЁС3МДПЩЕУЫљЧѓШШЛЏбЇЗНГЬЪНЁЃ
ЃН5ЁС1011 molЃЌЗХГіШШСП725.76 kJЁЄmolЃ1ЁС5ЁС1011 molЃН3.63ЁС1014 kJЃЛЃЈ3ЃЉЖдвбжЊШШЛЏбЇЗНГЬЪНБрКХДгЧАЕНКѓЗжБ№ЮЊЂйЂкЂлЃЌЂлЃЂкЃЋЂйЁС2МДПЩЕУЫљЧѓЗНГЬЪНЃЌЫљвдІЄHЃНЃЋ141 kJЁЄmolЃ1ЃЃЈЃ566 kJЁЄmolЃ1ЃЉЃЋЃЈЃ393.5 kJЁЄmolЃ1ЃЉЁС2ЃЛЃЈ4ЃЉЪзЯШаДГіЫљЧѓЗДгІЕФЛЏбЇЗНГЬЪН2O3ЃЈgЃЉ=3O2ЃЈgЃЉЃЌЧАЪНЁС2ЃЋКѓЪНЁС3МДПЩЕУЫљЧѓШШЛЏбЇЗНГЬЪНЁЃ