��Ŀ����
19��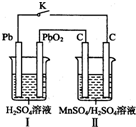 �̼��仯����Ӧ��Խ��Խ�㷺��MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�MnO2�ķ���֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��ӦʽΪMn2+-2e-+2H20=MnO2+4H+��
�̼��仯����Ӧ��Խ��Խ�㷺��MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�MnO2�ķ���֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��ӦʽΪMn2+-2e-+2H20=MnO2+4H+������Ǧ����Ϊ��Դ����ữ��MnS04��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪPb+Pb02+2H2S04=2PbSO4+2H20������������4mol H+������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ2mol��MnO2�����۲���Ϊ87g��
CaSO4��һ�������ʣ���֪Ksp��CaSO4��=9.10��10-6���ֽ�c mol•L-1 CaCl2��Һ��2.00��10-2 mol•L-1 Na2SO4��Һ�����������ɳ�������c����Сֵ��1.82��10-3���������3λ��Ч���֣���
���� II��������Mn2+�ŵ緢��������Ӧ������MnO2��ԭ���I�У�Pbʧ������������Pb02��������Pbʧ������������Pb02�õ��Ӷ�����������ӷ�Ӧ��������Ǧ�����������Ӻ�ת�Ƶ���֮��Ĺ�ϵʽ����ת�Ƶ��ӵ����ʵ���������ת�Ƶ�����ȼ���������̵����������ݳ����ܽ�ƽ����ܶȻ������������㣮
��� �⣺II��������Mn2+�ŵ緢��������Ӧ������MnO2��ͬʱ���������ӣ��缫��ӦʽΪMn2+-2e-+2H20=MnO2+4H+��
ԭ���I�У�Pbʧ������������Pb02��������Pbʧ������������Pb02�õ��Ӷ�����������ӷ�Ӧ��������Ǧ����ط�ӦʽΪPb+Pb02+2H2S04=2PbSO4+2H20��
�裺������4mol������ʱת�Ƶ��ӵ����ʵ���Ϊx��
Pb+Pb02+4H++S042-=2PbSO4+2H20ת�Ƶ���
4mol 2mol
4mol x
4mol��2mol=4mol��x
x=$\frac{4mol��2mol}{4mol}$=2mol��
������·��ת�Ƶ�����ȣ���ת��2mol����ʱ���ɶ������̵�����Ϊy��
Mn2+-2e-+2H20=MnO2+4H+��
2mol 87g
2mol y
2mol��87g=2mol��y
y=$\frac{87g��2mol}{2mol}$=87g
�ֽ�c mol•L-1CaCl2��Һ��2.00��10-2 mol•L-1 Na2SO4��Һ�����������ɳ�������Һ��c��Ca2+��=$\frac{c}{2}$mol/L��c��SO42-��=1.00��10-2mol/L��CaSO4��һ�������ʣ���֪KSP��CaSO4��=c��Ca2+��c��SO42-��=$\frac{c}{2}$mol/L��1.00��10-2mol/L=9.10��10-6��c=1.82��10-3mol/L��
�ʴ�Ϊ��Mn2+-2e-+2H20=MnO2+4H+��Pb+Pb02+2H2S04=2PbSO4+2H20��2 mol��87��1.82��10-3��
���� ���⿼��ԭ��غ͵���ԭ���Լ��ܶȻ��ļ��㣬���ؿ���缫��Ӧʽ����д������ʽ�ļ��㣬��ȷ�����缫�Ϸ����ķ�Ӧ�ǽⱾ��ؼ���֪��������·��ת�Ƶ����ص㣬��Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�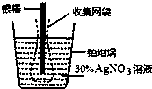 ��ͼΪ���ݵ��ԭ���Ƴɡ��������ơ�����ԭ���ǣ�ͨ���ⶨ�������и����ڶ��Ե缫�Ͻ�������������ͨ�����صĵ���������˵����ȷ���ǣ�������
��ͼΪ���ݵ��ԭ���Ƴɡ��������ơ�����ԭ���ǣ�ͨ���ⶨ�������и����ڶ��Ե缫�Ͻ�������������ͨ�����صĵ���������˵����ȷ���ǣ�������| A�� | �����ƹ���ʱ��Һ����������������ƶ� | |
| B�� | �����ƹ���ʱ����Ӧ���Դ���������� | |
| C�� | �������ռ����ܽ�����в����Ľ�����������û�и����������������ƫ�� | |
| D�� | ���ý������ij�����Ϊ1.08g�����������ת�Ƶĵ���Ϊ0.01mol |
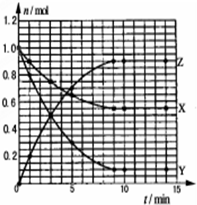 ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯��ͼ��ʾ��������˵��������ǣ�������
ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯��ͼ��ʾ��������˵��������ǣ�������| A�� | ��Ӧ�Ļ�ѧ����ʽ��X+2Y?2Z | |
| B�� | �÷�Ӧ��0-3minʱ���ڲ���Z��ƽ����Ӧ����0.083mol•L-1•min-1 | |
| C�� | ��ͼ��֪�÷�Ӧ�������� | |
| D�� | �����������䣬�����¶ȣ�������Ӧ�����������淴Ӧ���ʽ���С |
| A�� | 15g����-CH3�������еĵ�������10NA | |
| B�� | ���³�ѹ�£�30g��ȩ�����е�ԭ����ĿΪ4NA | |
| C�� | ��״���£�1L������ȼ�պ����ɵ���̬����ķ�����Ϊ5/22.4 NA | |
| D�� | ���³�ѹ�£�1mol���������еĹ��ۼ���ĿΪ12NA |
| A�� | NH4HCO3���ڹ�����ŨKOH��Һ�У�NH4++HCO3-+2OH-=CO32-+NH3��+2 H2O | |
| B�� | ��Ũ������鰱��NH3+HCl=NH4Cl | |
| C�� | ��������������ʴʱ������������������Fe-3e-=Fe3+ | |
| D�� | ��ͭ���缫���CuSO4��Һ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+ |
| A�� | BOH����ˮ������뷽��ʽ��BOH�TB++OH- | |
| B�� | ����һ������������Һ��Ϻ�pH=7����c��A- ��=c��B+�� | |
| C�� | ��0.1 mol/L BA��Һ�У�c��B+����c��A- ����c��OH- ����c��H+�� | |
| D�� | ����0.1 mol/L BOH��Һϡ����0.001 mol/L������Һ��pH=9 |
| A�� | ��ú������ȼ�� | |
| B�� | �ô�п�����п��ͬŨ��ͬ��������ᷴӦ������ | |
| C�� | ��ʳ�������ڱ����� | |
| D�� | ��˫��ˮ��Һ������ʱ���������������̷�ĩ |
| A�� | S��s��+O2��g����SO2��g����H1 S��g��+O2��g����SO2��g����H2 | |
| B�� | 2H2��g��+O2��g����2H2O��l����H 1 2H2��g��+O2��g����2H2O��g����H2 | |
| C�� | NaOH��aq��+HCl��aq����NaCl��aq��+H2O��l����H1 NaOH��aq��+CH3COOH��aq����CH3COONa��aq��+H2O��l����H2 | |
| D�� | H2��g��+F2��g����2HF��g����H1 H2��g��+Cl2��g����2HCl��g����H2 |
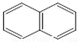 ��
�� 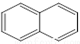 �������ǵ�ͬ�ģ�����[��]����ǿ�°����ʣ��������̴ѻҡ�ú���͡�ȼ�յ���������ȼ����β���У������ķ�����5���������϶��ɣ���ṹ���Ա�ʾΪ����ʽ��������Ҳ�ǵ�ͬ�ģ�
�������ǵ�ͬ�ģ�����[��]����ǿ�°����ʣ��������̴ѻҡ�ú���͡�ȼ�յ���������ȼ����β���У������ķ�����5���������϶��ɣ���ṹ���Ա�ʾΪ����ʽ��������Ҳ�ǵ�ͬ�ģ�