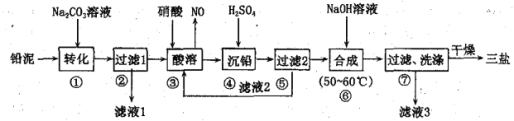
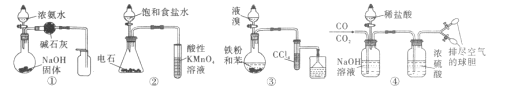
��Ŀ����
����Ŀ�����й��ھ����˵����ȷ������ǣ� ��
�ٷ��Ӿ����ж����ڹ��ۼ�
���ھ�����ֻҪ�������Ӿ�һ����������
�۽��ʯ��SiC��NaF��NaCl��H2O��H2S������۵����ν���
�����Ӿ�����ֻ�����Ӽ�û�й��ۼ������Ӿ����п϶�û�����Ӽ�
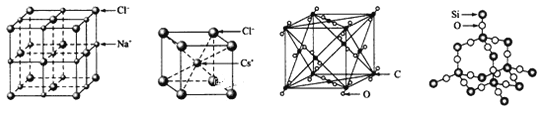
��CaTiO3������(�����ṹ��ͼ��ʾ)ÿ��Ti4+��12��O2-�����
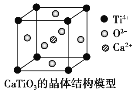
(ͼ��Ca2+��O2-��Ti4+�ֱ�λ������������ġ����ĺͶ���)
��SiO2������ÿ����ԭ����������ԭ���Թ��ۼ�����
�߾����з��Ӽ�������Խ����Խ�ȶ�
���Ȼ����ۻ�ʱ���Ӽ����ƻ�
A.�٢ڢܢ�B.�٢ڢ�C.�ۢݢ�D.�ۢݢ�
���𰸡�C
��������
��ϡ������Ϊ��ԭ�ӷ��ӣ������ڹ��ۼ����ʢٴ���
�ڽ��������������������ɵ��ӹ��ɣ�û�������ӣ��ʢڴ���
�۽��ʯ��SiC����ԭ�Ӿ��壬����C-C��Si-C���ʽ��ʯ�л�ѧ�����ȶ������۵���ߣ�NaF��NaCl��Ϊ���Ӿ��壬F-�뾶��С��NaF�ľ����ܸ����۵���ߣ�H2O��H2S�����ڷ��Ӿ��壬ˮ����֮�����������۵�ϸߣ��۵�ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬�ʽ��ʯ��SiC��NaF��NaCl��H2O��H2S������۵����ν��ͣ��ʢ���ȷ��
�ܹ��ۻ�����һ���������Ӽ��������Ӿ����п��ܺ��й��ۼ������������ơ��������ơ���εȣ��ʢܴ���
���Զ���Ti4+�����о�����֮�����O2-λ�������ϣ�ÿ��Ti4+����Ϊ12���湲�ã���ÿ��Ti4+��12��O2-����ڣ��ʢ���ȷ��
��SiO2������ÿ����ԭ�����ĸ���ԭ���γɹ��ۼ����ʢ���
�߷��Ӽ�������Ӱ���������ʣ������ȶ������ڻ�ѧ���ʣ��ʢߴ���
���Ȼ����������Ӿ��壬��֮������Ϊ���Ӽ����ۻ�ʱ�ƻ����Ӽ����ʢ���ȷ��
�ʴ�ΪC��