��Ŀ����
����Ŀ�������������ѧ��ѧ���õ��Լ�����Ҫ���ڷ�������������ҩ�ȡ�ʵ����ģ�ҵ�������̿��Ʊ�������ص��������£�
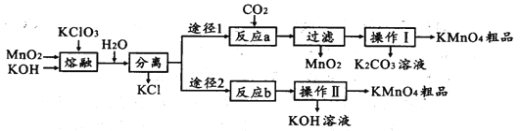
��1��ʵ�������ڶ������̡��������ء������ʱӦѡ����һ������________________
a.��ͨ�������� b.ʯӢ���� c.�մ����� d.������
��2����һ������ʱ����K2MnO4�Ļ�ѧ����ʽ��_________
��3���������и���KMnO4��K2CO3��������____________ (������)�ϵIJ��죬����Ũ���ᾧ�����ȹ��˵õ�KMnO4�����ȹ��˵�ԭ����_______________
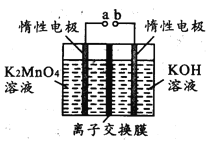
��4����Ӧb�ǵ�ⷨ�Ʊ�KMnO4����װ����ͼ��ʾ��a��____________��(����������������)���м�����ӽ���Ĥ��_____ (����������������)���ӽ���Ĥ�������ĵ缫��ӦʽΪ____________
��5��KMnO4ϡ��Һ��һ�ֳ��õ���������������ԭ��������������ͬ��________(����)��
a.˫��ˮ b.84��Һ(NaClO��Һ) c.75���ƾ�
���𰸡�d 3MnO2+KClO3+6KOH![]() 3K2MnO4+KCl+3H2O �ܽ��� �����¶��½����������Ʒ�Ĵ��Ƚ��� �� �� MnO42--e-=MnO4- ab
3K2MnO4+KCl+3H2O �ܽ��� �����¶��½����������Ʒ�Ĵ��Ƚ��� �� �� MnO42--e-=MnO4- ab
��������
�������̣������������������ط����ڿ��������ڣ�3MnO2+KClO3+6KOH ![]() 3K2MnO4+KCl+3H2O����ˮ�ܽ�����ȥKCl�õ�K2MnO4��Һ��
3K2MnO4+KCl+3H2O����ˮ�ܽ�����ȥKCl�õ�K2MnO4��Һ��
;��1����K2MnO4��Һͨ�������̼�õ�KMnO4��MnO2��K2CO3��3K2MnO4+2CO2=2KMnO4+2K2CO3+MnO2�������˳�ȥ����(MnO2)����ҺΪKMnO4��K2CO3��Һ��Ũ���ᾧ�����ȹ��˵õ�KMnO4���壬��Һ�к���K2CO3��
;��2�����K2MnO4��Һ��2K2MnO4+2H2O ![]() 2KMnO4+2KOH+H2����aΪ�������缫��ӦΪ��MnO42--e-=MnO4-���ݴ˷�������
2KMnO4+2KOH+H2����aΪ�������缫��ӦΪ��MnO42--e-=MnO4-���ݴ˷�������
(1)ʵ�������ڶ������̡��������ء������ʱӦѡ������������Ϊ��ͨ����������ʯӢ�������մ������к��ж������裬����KOH��Ӧ���ʴ�Ϊd��
(2)��һ������ʱ�������̡��������ء����������K2MnO4�Ļ�ѧ����ʽΪ��3MnO2+KClO3+6KOH![]() 3K2MnO4+KCl+3H2O��
3K2MnO4+KCl+3H2O��
(3)����Ũ���ᾧ�����ȹ��˵õ�KMnO4��˵��������ص��ܽ������¶�Ӱ��ϴ������и���KMnO4��K2CO3���������ܽ����ϵIJ��죬����Ũ���ᾧ�����ȹ��˵õ�KMnO4�����ȹ��˵�ԭ���DZ����¶��½����������Ʒ���Ƚ��ͣ�
(4)��Ӧb�ǵ�ⷨ�Ʊ�KMnO4��aΪ������K2MnO4����KMnO4��������Ӧ��MnO42--e-=MnO4-������Ϊb���������ӷŵ磬�õ�KOH�������ӽ���ĤΪ�����ӽ���Ĥ�����������ӣ�
(5)KMnO4ϡ��Һ��Ϊ��ǿ��������һ�ֳ��õ���������˫��ˮ��84��Һ(NaClO��Һ)���������ԣ����������ʴ�Ϊ��ab��

����Ŀ�����к͵ζ���ԭ����ʵ������������Ӧ�ù㷺����I2O5�ɶ����ⶨCO�ĺ������÷�Ӧԭ��Ϊ5CO+I2O5![]() 5CO2+I2����ʵ�鲽�����£�
5CO2+I2����ʵ�鲽�����£�
��ȡ250 mL����״��������CO��ij������Ʒͨ��ʢ������I2O5�ĸ��������170 ���³�ַ�Ӧ��
����ˮһ�Ҵ�Һ����ܽ����I2������100 mL��Һ��
����ȡ���������Һ25.00 mL����ƿ�У�Ȼ����0.01 mol��L-1��Na2S2O3����Һ�ζ������ı�Na2S2O3��Һ����������ʾ��
��һ�� | �ڶ��� | ������ | |
�ζ�ǰ����/mL | 2.10 | 2.50 | 1.40 |
������/mL | 22.00 | 22.50 | 21.50 |
��1�������������100 mL������Һ��Ҫ�õ��IJ����������������ձ�����Ͳ������������ͷ�ιܺ�____________________��
��2��Na2S2O3��ҺӦװ��__________������ĸ���С�

��3��ָʾ��Ӧѡ��__________���жϴﵽ�ζ��յ��������____________________________________��
��4��������Ʒ��CO���������Ϊ__________����֪��������Ʒ�������ɷֲ���I2O5��Ӧ��2Na2S2O3+I2=2NaI+Na2S4O6��
��5�����в������������CO���������ƫ�����__________������ĸ����
a���ζ��յ㸩�Ӷ���
b����ƿ�ô�����Һ��ϴ
c���ζ�ǰ�����ݣ��ζ���û������
d������100 mL������Һʱ������������