��Ŀ����
4����A��B��C��D����ǿ����ʣ�������ˮ�е���ʱ�ɲ����������ӣ�ÿ������ֻ��һ�������Ӻ�һ���������һ����ظ���| ������ | K+��Na+��Ba2+��NH4+ |
| ������ | CH3COO-��Cl-��OH-��SO42- |
��A��C��Һ��pH������7��B��Һ��pHС��7��A��B����Һ��ˮ�ĵ���̶���ͬ��D��Һ��ɫ��Ӧ������ɫ�ܲ���������ɫ��
��C��Һ��D��Һ����ʱֻ���ɰ�ɫ������B��Һ��C��Һ����ʱֻ���ɴ̼�����ζ�����壬A��Һ��D��Һ���ʱ����������
��1��A�Ļ�ѧʽ��CH3COONa��
��2�������ӷ���ʽ��ʾB��ˮ��ҺpHС��7ԭ��NH4++H2O?NH3•H2O+H+��
��3��д��C��Һ��D��Һ��Ӧ�����ӷ���ʽBa2++SO42-=BaSO4����
��4��pH=10��A��Һ��pH=10��C��Һ��ˮ�ĵ���̶ȴ����CH3COONa����дA��C�Ļ�ѧʽ����
��5����������������ʵ���Ũ�ȵ�B��Һ��C��Һ��ϣ���Ӧ����Һ�и�������Ũ���ɴ�С��˳��Ϊc��OH-����c��Ba2+��=c��Cl-����c��NH4+����c��H+����
���� A��C��Һ��pH������7��ӦΪ�����κͼ���Һ��B��Һ��pHС��7��ӦΪ�����Һ��A��B����Һ��ˮ�ĵ���̶���ͬ������Һˮ��̶���ͬ��D��Һ��ɫ��Ӧ������ɫ�ܲ���������ɫ����Һ�к��м����ӣ�����AΪ�����ƣ�C��Һ��D��Һ����ʱֻ���ɰ�ɫ������B��Һ��C��Һ����ʱֻ���ɴ̼�����ζ�����壬A��Һ��D��Һ���ʱ������������˵��CΪBa��OH��2��DΪNa2SO4����BΪNH4Cl������������A��CH3COONa��B��NH4Cl��C��Ba��OH��2��D��K2SO4���ݴ˴��⣮
��� �⣺A��C��Һ��pH������7��ӦΪ�����κͼ���Һ��B��Һ��pHС��7��ӦΪ�����Һ��A��B����Һ��ˮ�ĵ���̶���ͬ������Һˮ��̶���ͬ��D��Һ��ɫ��Ӧ������ɫ�ܲ���������ɫ����Һ�к��м����ӣ�����AΪ�����ƣ�C��Һ��D��Һ����ʱֻ���ɰ�ɫ������B��Һ��C��Һ����ʱֻ���ɴ̼�����ζ�����壬A��Һ��D��Һ���ʱ������������˵��CΪBa��OH��2��DΪNa2SO4����BΪNH4Cl������������A��CH3COONa��B��NH4Cl��C��Ba��OH��2��D��K2SO4��
��1����������ķ�����֪��A�Ļ�ѧʽΪ��CH3COONa��
�ʴ�Ϊ��CH3COONa��
��2��BΪNH4Cl��笠�����ˮ��ʹ��Һ�����ԣ������ӷ���ʽ��ʾΪNH4++H2O?NH3•H2O+H+��
�ʴ�Ϊ��NH4++H2O?NH3•H2O+H+��
��3��C��Һ��D��Һ��Ӧ�����ӷ���ʽΪBa2++SO42-=BaSO4����
�ʴ�Ϊ��Ba2++SO42-=BaSO4����
��4��CH3COONaˮ��ٽ�ˮ�ĵ��룬Ba��OH��2����ˮ�ĵ��룬����c��H+��=10-10mol•L-1��CH3COOK��ˮ�������c��OH-��=10-4 mol•L-1��Ba��OH��2��Һ��ˮ�������
c��OH?��=c��H+��=10-10mol•L-1����Ba��OH��2��ˮ����̶�С��
�ʴ�Ϊ��CH3COONa��
��5����������֪���Ȼ�狀��������������ʵ�����ȣ���Ϻ���Һ�е������ǰ�ˮ���Ȼ����������������Ȼ���������������Ũ����ȣ���ˮ��Ũ�����Ȼ�������������Ũ�ȵ�2������Һ������������Ũ��������Ӻ�������Ũ����ȣ���ˮ������ֵ��뵼��������Ũ�ȴ���笠�����Ũ�ȣ���Һ�ʼ��ԣ�������Ũ����С��������Һ������Ũ�ȴ�С˳����c��OH-����c��Ba2+��=c��Cl-����c��NH4+����c��H+����
�ʴ�Ϊ��c��OH-����c��Ba2+��=c��Cl-����c��NH4+����c��H+����
���� ���⿼��������ƶϣ���Ŀ��Ϊ�ۺϣ��Ѷ��еȣ���ȷ�ƶϸ�������Ϊ�����Ĺؼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 100 mL 2 mol/L������п��Ӧʱ����п�۴���п�����������������ʲ��� | |
| B�� | ���ڷ�Ӧ2CO+2NO?N2+2CO2��ʹ�ú��ʵĴ�����CO���������ʺ��������ʶ��ӿ� | |
| C�� | ��������Ĵ�������һ�����ȷ�Ӧ�������¶ȣ���Ӧ���ʼ��� | |
| D�� | ����Ƭ��ϡ���ᷴӦ��ȡ����ʱ����ϡ�����ΪŨ������Լӿ��������������� |
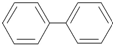 �����Ķ��ȴ��ﹲ�м��֣������ǿռ������칹����������
�����Ķ��ȴ��ﹲ�м��֣������ǿռ������칹����������| A�� | 10�� | B�� | 11�� | C�� | 12�� | D�� | 15�� |
| A�� | ��ԭ�Ӻ�����13�ֲ�ͬ�˶�״̬�ĵ��� | |
| B�� | ǰ������Ԫ���У���̬ԭ���гɶԵ���������������������ͬ��Ԫ����6�� | |
| C�� | ��һ�����ܽ���B��N֮��ĵڶ�����Ԫ����3�� | |
| D�� | HF��ˮ��Һ�д���4����� |

| A�� | a��Ϊ��صĸ������õ缫����������Ӧ | |
| B�� | ��ع���ʱ������a�������ص��߾����ݵ�b�� | |
| C�� | ��������ĵ缫��ӦʽΪ O2+2H2O+4e-�T4OH- | |
| D�� | ��ع���ʱ��1mol�Ҵ�������ת��12mol���� |
| A�� | v ��NH3��=0.2 mol/��L•s�� | B�� | v ��O2��=1.4mol/��L•min�� | ||
| C�� | v ��H2O��=0.25 mol/��L•s�� | D�� | v ��NO��=0.9 mol/��L•min�� |


 ��
��