��Ŀ����
10��A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ������������ԭ�Ӱ뾶��B��A��E��D��C��������C��B�ɰ�ԭ�Ӹ�����2��l��1��1�ֱ��γ��������ӻ�������ң�Aԭ�ӵ������������ȴ�����������3����E�ǵؿ��к�����ߵĽ���Ԫ�أ�����������Ϣ�ش��������⣺��1��DԪ�������ڱ��е�λ���ǵ������ڵڢ�A��
��2�������ʵĵ���ʽ��
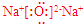
��3��E�ĵ��ʼ��뵽C������������Ӧ��ˮ�������Һ�з�Ӧ�����ӷ���ʽ��2 Al+2OH-+2H2O=2AlO2-+3H2��
��4���õ���ʽ��ʾA����̬�⻯����γɹ���
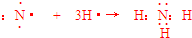 ��
��
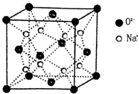
���� A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������ԭ�Ӱ뾶��B��A��E��D��C���������ݵ��Ӳ���Խ�࣬�뾶Խ���Ӳ�����ͬʱ�˵����Խ�࣬�뾶ԽС��֪A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڣ���A��Bλ�ڵڶ����ڣ�C��D��E���ڵ������ڣ�C��B�ɰ�ԭ�Ӹ�����2��l��1��1�ֱ��γ��������ӻ�������ң���C��NaԪ�ء�B��OԪ�أ�����Na2O������Na2O2��Aԭ�ӵ������������ȴ�����������3�����������2�����ӣ������������3�����ӣ���A��NԪ�أ�E�ǵؿ��к�����ߵĽ���Ԫ�أ���E��AlԪ�أ�D��ԭ����������C��С��E����D��MgԪ�أ��ٽ����Ŀ�������
��� �⣺A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������ԭ�Ӱ뾶��B��A��E��D��C���������ݵ��Ӳ���Խ�࣬�뾶Խ���Ӳ�����ͬʱ�˵����Խ�࣬�뾶ԽС��֪A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڣ���A��Bλ�ڵڶ����ڣ�C��D��E���ڵ������ڣ�C��B�ɰ�ԭ�Ӹ�����2��l��1��1�ֱ��γ��������ӻ�������ң���C��NaԪ�ء�B��OԪ�أ�����Na2O������Na2O2��Aԭ�ӵ������������ȴ�����������3�����������2�����ӣ������������3�����ӣ���A��NԪ�أ�E�ǵؿ��к�����ߵĽ���Ԫ�أ���E��AlԪ�أ�D��ԭ����������C��С��E����D��MgԪ�أ�
��1��D��MgԪ�أ�ԭ�Ӻ�����3�����Ӳ㡢�������2�����ӣ�����λ�ڵ������ڵ�IIA�壬
�ʴ�Ϊ���������ڵڢ�A�壻
��2������Na2O�������ʽΪ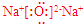 ��
��
�ʴ�Ϊ��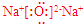 ��
��
��3�������뵽C������������Ӧ��ˮ���T�������Ƶ���Һ�з�Ӧ�����ӷ���ʽ��Ϊ2 Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2 Al+2OH-+2H2O=2AlO2-+3H2����
��4��A����̬�⻯��Ϊ�������õ���ʽ��ʾ���γɹ���Ϊ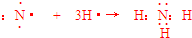 ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼����Ԫ�����ڱ���Ԫ�������ɵ�Ӧ�ã���ϤԪ�����ڱ��ṹ��Ԫ���������ں��ǽⱾ��ؼ���֪��ͬһ���塢ͬһ����Ԫ��������֪ʶ�����������֪ʶ������⣮

| A�� | NH4HCO3������ķ�Ӧ�Ƿ��ȷ�Ӧ | |
| B�� | �÷�Ӧ�У�����ת��Ϊ�����ڲ������� | |
| C�� | ��Ӧ�������������������������� | |
| D�� | ���Ѿɻ�ѧ�����յ�������С���γ��»�ѧ���ų��������� |
| ������ | K+��Na+��Ba2+��NH4+ |
| ������ | CH3COO-��Cl-��OH-��SO42- |
��A��C��Һ��pH������7��B��Һ��pHС��7��A��B����Һ��ˮ�ĵ���̶���ͬ��D��Һ��ɫ��Ӧ������ɫ�ܲ���������ɫ��
��C��Һ��D��Һ����ʱֻ���ɰ�ɫ������B��Һ��C��Һ����ʱֻ���ɴ̼�����ζ�����壬A��Һ��D��Һ���ʱ����������
��1��A�Ļ�ѧʽ��CH3COONa��
��2�������ӷ���ʽ��ʾB��ˮ��ҺpHС��7ԭ��NH4++H2O?NH3•H2O+H+��
��3��д��C��Һ��D��Һ��Ӧ�����ӷ���ʽBa2++SO42-=BaSO4����
��4��pH=10��A��Һ��pH=10��C��Һ��ˮ�ĵ���̶ȴ����CH3COONa����дA��C�Ļ�ѧʽ����
��5����������������ʵ���Ũ�ȵ�B��Һ��C��Һ��ϣ���Ӧ����Һ�и�������Ũ���ɴ�С��˳��Ϊc��OH-����c��Ba2+��=c��Cl-����c��NH4+����c��H+����
| X | ||
| M |
�ң�g��+Z2��g�����ף�g��+Q2��g����ÿ����1mol Q2����57.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��2HCl��g��+$\frac{1}{2}$O2��g��=H2O��g��+Cl2��g����H=-57.8 kJ/mol��4HCl��g��+O2��g��=2H2O��g�� 2Cl2��g����H=-115.6 kJ/mol��
��2��Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ���������ˮ��Һ�����ԣ���ԭ���ǣ������ӷ���ʽ˵����NH4++H2O?NH3��H2O+H+������Һ�и�������Ũ���ɴ�С��˳��Ϊc��NO3-����c��NH4+����c��H+����c��OH-����
��3��X��Y�ɷֱ���Z��ԭ�Ӹ�����1��1�γɻ�������Ͷ����ڴ������������£����붡�������淴Ӧ�����ɵ���Y2����һ�ֻ������죮д���÷�Ӧ�Ļ�ѧ����ʽ��
2CO+2NO
 N2+2CO2���÷�Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��C{O}_{2}��•c��{N}_{2}��}{{c}^{2}��CO��•{c}^{2}��NO��}$��
N2+2CO2���÷�Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��C{O}_{2}��•c��{N}_{2}��}{{c}^{2}��CO��•{c}^{2}��NO��}$����4����1mol�������1mol���������װ�д����ĺ�ѹ�����У���ַ�Ӧ��ƽ��������������ԭ������23%����ת����Ϊ92%��
����֪�÷�Ӧ��H��0���������¶ȣ�ƽ�ⳣ��Kֵ����С������С�䣩��
��������ѹǿ����Ϊԭ����2�����ٴδ�ƽ���������ж��������������С������С�䣩��
��5����X�������̬�⻯�Z�ĵ��ʺ�KOH��Һ��ɵ�����ȼ�ϵ���У������Ϸ�����Ӧ�ĵ缫��ӦʽΪCH4+10OH--8e-=CO32-+7H2O��
| ѡ�� | ʵ����ʵ | ���۽��� |
| A | ��ԭ�ӵĵ�һ�����ܴ�����ԭ�� | ��ԭ��2p�ܼ������ |
| B | CO2Ϊֱ���η��� | CO2������C-O�Ǽ��Լ� |
| C | ���ʯ���۵����ʯī | ���ʯ�Ƿ��Ӿ��壬ʯī��ԭ�Ӿ��� |
| D | HF�ķе����HCl | HF����Է�������С��HCl |
| A�� | A | B�� | B | C�� | C | D�� | D |
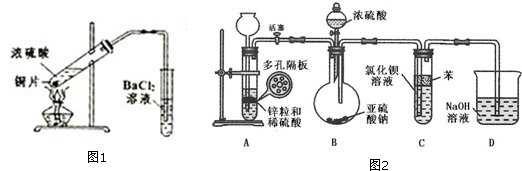
 ��
��
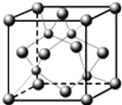 A��B��C��D����Ԫ�����ڱ��еĶ�����Ԫ�أ����ǵĺ˵������������2����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2����Bԭ�ӵ������p����ĵ���Ϊ�����ṹ��C�ǵؿ��к�������Ԫ�أ�Dԭ�ӵ�S������P��������ȣ�E�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ����������ö�Ӧ��Ԫ�ط��Ż�ѧʽ�ش��������⣺
A��B��C��D����Ԫ�����ڱ��еĶ�����Ԫ�أ����ǵĺ˵������������2����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2����Bԭ�ӵ������p����ĵ���Ϊ�����ṹ��C�ǵؿ��к�������Ԫ�أ�Dԭ�ӵ�S������P��������ȣ�E�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ����������ö�Ӧ��Ԫ�ط��Ż�ѧʽ�ش��������⣺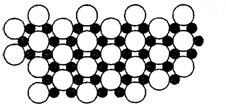
 ��1�����ɽ���Ԫ�������γɶ��������磺[Fe��H2NCONH2��6]��NO3��3[�����������غ�������Fe��CO��x�ȣ�
��1�����ɽ���Ԫ�������γɶ��������磺[Fe��H2NCONH2��6]��NO3��3[�����������غ�������Fe��CO��x�ȣ�