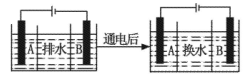
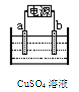
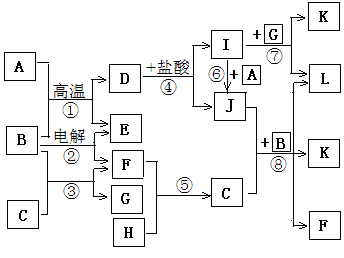
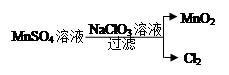��Ŀ����
�������ֿ���������A��B��C��D��E�������������������ӻ�����ͬ���ֱ�������������Na����Al3����Mg2����Ba2����Fe3��������������Cl����OH����NO3����CO32����X�е�һ�֡�
(1)ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������________��________(�ѧʽ)��
(2)Ϊ��ȷ��X���ֽ�(1)�е��������ʼ�ΪA��B����X�����ʼ�ΪC����C��B��Һ���ʱ���������ɫ��������ɫ��ζ���壻��C��A����Һ���ʱ�����ػ�ɫ��������ó����е���ϡ������������ܽ⣬������а�ɫ���������ܽ⡣��XΪ________��
A��SO32����������B��SO42�� C��CH3COO�� D��SiO32��
(3)��CuͶ�뵽װ��D��Һ���Թ��У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�������Dһ���������������е�________(����Ӧ�����ӷ���)���йط�Ӧ�����ӷ���ʽΪ___________________��
(4)���������Ѿ�ȷ�������ʣ����Լ����D��E�е������ӣ������ʵ��������衢�����ۣ�__________________________________________��
��(1)Na2CO3��Ba(OH)2��(2)B
(3)NO3����3Cu��8H����2NO3��=3Cu2����2NO����4H2O��(4)��D����Һ������Ba(OH)2��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬��D�к���Al3���������ɰ�ɫ�������ܽ⣬��D�к���Mg2��(����������Ҳ��)
����

ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪��
�����ԣ�IO3-��Fe3����I2����ԭ�ԣ�S2O32-��I��
3I2��6OH��=5I����IO3-��3H2O
KI��I2 KI3
KI3
(1)ijѧϰС��Լӵ��ν���������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ������ (�û�ѧʽ��ʾ)��CCl4�����Ϻ�ɫ�������� (�õ���ʽ��ʾ)��
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ �� ��
(2)KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ�� ��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ� (��ǡ���)����˵�����ɣ� ��
(3)Ϊ����ӵ���(����KI)���ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ������� ��
| A��Na2S2O3 | B��AlCl3 |
| C��Na2CO3 | D��NaNO2 |

 [Fe(SCN) ]2+��
[Fe(SCN) ]2+��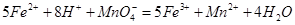 ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
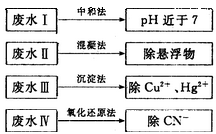
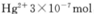 ���Ƿ�ﵽ���ŷű�_______����ǡ�����
���Ƿ�ﵽ���ŷű�_______����ǡ�����