��Ŀ����
��10�֣������Ϊ100 mLŨ��Ϊ2.0 mol?L?1 ��CuSO4��Һ�����Ϊ300 mLŨ��Ϊ1.0 mol?L?1 ��H2SO4��Һ��ϣ��ٶ���Ϻ���Һ���Ϊ400 mL�����㣺
��1�����Һ��CuSO4�����ʵ���Ũ���� ��H2SO4�����ʵ���Ũ���� ��
��2�������Һ�м�ˮϡ����Cu2+ Ũ��Ϊ0.2 mol?L?1����ϡ�ͺ���Һ�����Ϊ ��
��3��ϡ�ͺ���Һ��H+ �����ʵ���Ũ���� ��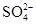 �����ʵ���Ũ���� ��
�����ʵ���Ũ���� ��
��1�����Һ��CuSO4�����ʵ���Ũ���� ��H2SO4�����ʵ���Ũ���� ��
��2�������Һ�м�ˮϡ����Cu2+ Ũ��Ϊ0.2 mol?L?1����ϡ�ͺ���Һ�����Ϊ ��
��3��ϡ�ͺ���Һ��H+ �����ʵ���Ũ���� ��
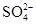 �����ʵ���Ũ���� ��
�����ʵ���Ũ���� ��(1) 0.5 mol?L?1 0.75 mol?L?1 ��2��1000mL (3)0.6 mol?L?1 0.3 mol?L?1
�����������1��n(CuSO4)=0.2mol n(H2SO4)=0.3mol����Ϻ���Һ�����Ϊ400mL������c(CuSO4)=0.5mol/L c(H2SO4)=0.75mol��
��2������c(ϡ)V(ϡ)=c(Ũ)V(Ũ)��0.2 mol?L?1��V=0.5mol/L��400mL����V=1000mL��
��3��n(H+)=0.6mol n(SO42-)=0.3mol����Һ���Ϊ1L����c(H+)=0.6mol/L��c(SO42-)=0.3mol/L��
����������ܻ������Ѷ�С���ʺ���ѵ��ѧ���Ļ���������

��ϰ��ϵ�д�
�����Ŀ