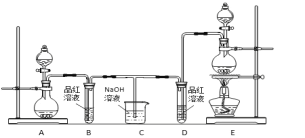
��Ŀ����
����Ŀ����ͬ�¶��£������Ϊ0.25 L �����������ܱ������з������淴Ӧ��N2(g)��3H2(g)![]() 2NH3(g) ��H����92.6kJ/mol.ʵ������ʼ��ƽ��ʱ���й����������
2NH3(g) ��H����92.6kJ/mol.ʵ������ʼ��ƽ��ʱ���й����������
������� | ��ʼʱ���������ʵ���/mol | ��ƽ��ʱ��ϵ�����ı仯 | ||
N2 | H2 | NH3 | ||
�� | 1 | 3 | 0 | �ų�������23.15kJ |
�� | 0.9 | 2.7 | 0.2 | �ų�������Q |
��������������ǣ� ��
A.�����١����з�Ӧ��ƽ�ⳣ�����
B.ƽ��ʱ������������NH3������������
C.�������д�ƽ��ʱ�ų�������Q��23.15kJ
D.�����������Ϊ0.5L����ƽ��ʱ�ų�������С��23.15kJ
���𰸡�C
��������
A. ƽ�ⳣ��ֻ���¶��йأ���ͬ�¶��£������Ϊ0.25L�����������ܱ������з�����ͬ�ķ�Ӧ����ƽ�ⳣ��Ӧ��ͬ����A��ȷ��
B. �������зų�23.15kJ�����������ɰ��������ʵ���Ϊ��![]() =0.5mol����������ʽ�����㣺
=0.5mol����������ʽ�����㣺
N2(g)+3H2(g)2NH3(g)��H=-92.6kJ/mol
��ʼ��1mol3mol0
ת����0.25mol0.75mol0.5mol
ƽ�⣺0.75mol2.25mol0.5mol
ƽ��ʱ����������NH3���������������������ʵ�������![]() ��
��
�ӵ�Чƽ��ĽǶȷ�����0.9molN2��2.7molH2��0.2molNH3�൱��1molN2��3molH2������ͬ�����´�����ͬƽ��״̬����Чƽ�⣬����ƽ��ʱ���������ڰ��������������ȣ���B��ȷ��
C. ���к���0.2molNH3�൱�ڢٵĻ����ϼ��백��������ƽ�������ƶ�����ƽ��ʱ�ų�������С��23.15kJ����C����
D. �����������Ϊ0.5L���൱����ԭ���Ļ����ϼ�Сѹǿ��ƽ�������ƶ���ƽ��ʱ�ų�������С��23.15kJ����D��ȷ��
��ѡC��

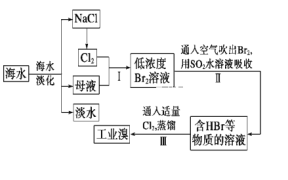
����Ŀ����Ȼ���д��ڴ�����Ԫ�أ����ơ�þ����������ͭ���顢п�����������������Ź㷺��Ӧ�á�
(1)��д��Fe�Ļ�̬ԭ�Ӻ�������Ų�ʽ________��������________��Ԫ�ء�
(2)����A��ԭ��ֻ��3�����Ӳ㣬���һ�����ĵ��������£�
������ |
|
|
|
|
A | 932 | 1821 | 15390 | 21771 |
��Aԭ�ӵļ۵����Ų�ʽΪ___________________________��
(3)п��ͭ��һ�ֽ���������仯ѧʽ�ж�����ʽ����![]() ��
��![]() ��
��![]() �ȡ���������Ԫ��ͭ�ĵڶ�������________(������������С��������������)п�ĵڶ������ܡ�
�ȡ���������Ԫ��ͭ�ĵڶ�������________(������������С��������������)п�ĵڶ������ܡ�
(4)��п��ԭ![]() ��������Һ���������������Ƶ���ɫ�ľ���
��������Һ���������������Ƶ���ɫ�ľ���![]() ���þ�������Ԫ���У��縺������Ԫ����________����Ti�γ���λ����������________��
���þ�������Ԫ���У��縺������Ԫ����________����Ti�γ���λ����������________��![]() ��������к���
��������к���![]() ������ĿΪ________��
������ĿΪ________��
(5)![]() ���γɶ��������ӣ���
���γɶ��������ӣ���![]() ��
��![]() �ȡ�
�ȡ�![]() �Ŀռ乹��Ϊ��________����
�Ŀռ乹��Ϊ��________����![]() ��Ϊ�ȵ�����ķ����У�___________________(�����ʽ)��
��Ϊ�ȵ�����ķ����У�___________________(�����ʽ)��
(6)ͭ�Ļ���������ܶ࣬��ͼ���Ȼ���ͭ�ľ����ṹ(��ɫ���ʾ![]() ����ɫ���ʾ
����ɫ���ʾ![]() )����֪�������ⳤΪacm�����Ȼ���ͭ�ܶȵļ���ʽΪ��=________
)����֪�������ⳤΪacm�����Ȼ���ͭ�ܶȵļ���ʽΪ��=________![]() (��
(��![]() ��ʾ�����ӵ�����)
��ʾ�����ӵ�����)