��Ŀ����
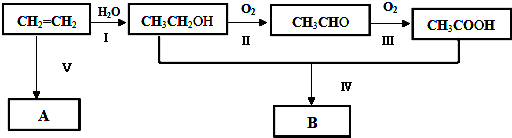
5��ͨ��ʯ���ѽ���Ի����ϩ��������ϩΪԭ�ϻ����Ժϳɺܶ�Ļ�����Ʒ���Ը�����ͼ�ش��й����⣨��ʾ��2CH3CHO+O2 $��_{��}^{����}$2CH3COOH����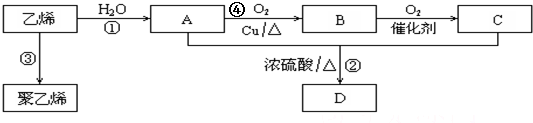
��1��д��A���ʵĽṹ��ʽCH3CH2OH��A��ͬ���칹��Ľṹ��ʽCH3OCH3��
��2��д�����з�Ӧ�ķ�Ӧ���ͣ��ټӳɷ�Ӧ����ȡ����Ӧ��������Ӧ���ۼӾ۷�Ӧ��
��3��д��ͼʾ��Ӧ�١��ۡ��ܵĻ�ѧ����ʽ��
��CH2=CH2+H2O$\stackrel{һ��������}{��}$CH3CH2OH��
��
 ��
����2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� C2H4�����Ӿ۷�Ӧ�õ�����ϩ����ϩ��ˮ�����ӳɷ�Ӧ����AΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��BΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��CΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����CH3COOCH2CH3����DΪCH3COOCH2CH3���ݴ˷������
��� �⣺C2H4�����Ӿ۷�Ӧ�õ�����ϩ����ϩ��ˮ�����ӳɷ�Ӧ����AΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��BΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��CΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����CH3COOCH2CH3����DΪCH3COOCH2CH3��
��1��A���Ҵ����ṹ��ʽΪCH3CH2OH��A��ͬ���칹��ΪCH3OCH3���ʴ�Ϊ��CH3CH2OH��CH3OCH3��
��2��ͨ�����Ϸ���֪���٢ڢ۷ֱ��Ǽӳɷ�Ӧ��������Ӧ��ȡ����Ӧ���Ӿ۷�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ���Ӿ۷�Ӧ��
��3������ϩ��ˮ�ӳɷ�Ӧ�����Ҵ�����Ӧ�ķ���ʽΪCH2=CH2+H2O$\stackrel{һ��������}{��}$CH3CH2OH��
��Ϊ��ϩ�ļӾ۷�Ӧ����Ӧ����ʽΪ ��
��
�ܸ÷�ӦΪ�Ҵ��Ĵ���������Ӧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{һ��������}{��}$CH3CH2OH�� ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ��������漰ϩ������ȩ�����ᡢ��֮���ת����ע���Ϸ�Ӧ���������ƶϣ�֪���Ҵ��Ĵ������ϼ���ʽ����Ŀ�ѶȲ���

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�| A�� | �ɼ�����ȴ�����4�֣�����֪������ȴ�����6�� | |
| B�� | ��ϩ�ͱ���������ʹ��ˮ��ɫ�����ܼ�����ϩ�ͱ����� | |
| C�� | ���ۡ���֬��������һ�������¶��ܷ���ˮ�� | |
| D�� | ��ϩ�;���ϩ����ʹ������Ȼ�̼��Һ��ɫ |
| A�� | ������SO2ͨ�뺬Fe2+��Cl-��Ba2+��Al3+����Һ�У������������ܴ������� | |
| B�� | ��NaClO��Һ��ͨ������������̼�����ӷ���ʽ��2ClO-+CO2+H2O�T2HClO+CO32- | |
| C�� | ����������������HBr��Һ��Ӧ�����ӷ���ʽ��Fe��OH��2+3H+�TFe2++3H2O | |
| D�� | ��100mL1mol•L-1��FeCl3��Һ������NaS�����ַ�Ӧ�����ɳ���10.4g |
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� |
��2����ЩԪ�ص����������Ķ�Ӧˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������ǿ�Ļ�����ĵ���ʽ��
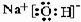 �����γ��������������Ԫ����Al��
�����γ��������������Ԫ����Al����3���ڵ��⻯�����ʽΪNH3�����ڹ��ۻ��������ۡ������ӡ�����
��4����Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
 ��
�� 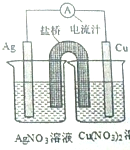 ijͬѧ��ͭƬ����Ƭ��Cu��NO3��2��Һ��AgNO3��Һ�����ߺ����ţ�װ����֬-KN03��U�ιܣ����һ��ԭ�����ͼ��ʾ�������ж�����ȷ���ǣ�������
ijͬѧ��ͭƬ����Ƭ��Cu��NO3��2��Һ��AgNO3��Һ�����ߺ����ţ�װ����֬-KN03��U�ιܣ����һ��ԭ�����ͼ��ʾ�������ж�����ȷ���ǣ�������| A�� | ʵ������У����������ձ��У�NO3-Ũ�ȱ仯����ֱ�Ϊ���䣬���� | |
| B�� | ʵ������У�ȡ�����ţ���ԭ���Ҳ�ܼ������� | |
| C�� | ����ʼʹ��U��ͭ�ܴ������ţ�װ�����е���������ͭ���������� | |
| D�� | ����ʼʱ��U��ͭ�ܴ������ţ�װ������������ |