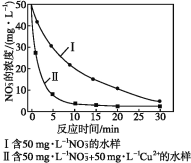
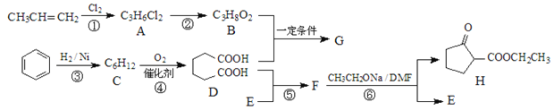
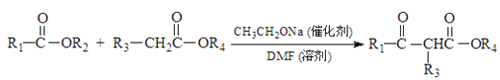
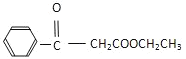��Ŀ����
����Ŀ������������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ��ͼ��ʾ��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T���γɻ�����Z2T�������ƶ���ȷ���ǣ� ��

A.�����Ӱ뾶��T>Z>Y
B.Z2Y��Z2Y2�����еĻ�ѧ��������ͬ
C.����X2Y�ķе����X2T�����Ƴ�X2Y���ȶ���ǿ��X2T
D.ZXT��ˮ��Һ�������ԣ��ٽ���ˮ�ĵ���
���𰸡�D
��������
Rԭ�������������ǵ��Ӳ�����2����R����ΪCԪ�ػ�SԪ�أ�����ͼʾԭ�Ӱ뾶��ԭ��������ϵ��֪RӦΪCԪ�أ�Y��Z���γ�Z2Y��Z2Y2�����ӻ����ӦΪNa2O��Na2O2����YΪOԪ�أ�ZΪNaԪ�أ�Z��T�γɵ�Z2T�������T��ԭ�Ӱ뾶��NaС��ԭ������T>Z����TӦΪSԪ�أ�X��ԭ�Ӱ뾶��С��ԭ��������С��ԭ�Ӱ뾶Ҳ��С����XӦΪHԪ�أ���϶�Ӧ���ʡ�������������Լ���ĿҪ������⡣
���������ƶϿ�֪��X��HԪ�أ�Y��OԪ�أ�Z��NaԪ�أ�R��CԪ�أ�T��SԪ�ء�
A.S2-������3�����Ӳ㣬O2-��Na+������2�����Ӳ㣬�������Ӻ�����Ӳ���Խ�࣬���Ӱ뾶Խ�����Ӻ�����Ӳ�����ͬʱ���˵����Խ�����Ӱ뾶ԽС���������Ӱ뾶��T>Y>Z��A����
B. Z2Y��Z2Y2��ʾ����Na2O��Na2O2��Na2Oֻ�����Ӽ���Na2O2�������Ӽ������Թ��ۼ�����������Ļ�ѧ�����Ͳ���ͬ��B����
C.O��S��ͬһ�����Ԫ�أ�����H2O����֮������������H2S����֮��ֻ���ڷ��»���������Ĵ���ʹH2O�ķе��H2S�ߣ������ʵ��ȶ���������ڵĹ��ۼ���ǿ���йأ�����Ӽ���������С�أ�C����
D.ZXT��ʾ����NaHS��������Ϊǿ�������Σ�����Һ��HS-ˮ�⣬����ˮ���������H+����γ�H2S��ʹˮ�ĵ���ƽ�������ƶ����ٽ���ˮ�ĵ��룬ʹ��Һ��c(OH-)�������մﵽƽ��ʱ����Һ��c(OH-)>c(H+)����Һ�Լ��ԣ�D��ȷ��
�ʺ���ѡ����D��

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�