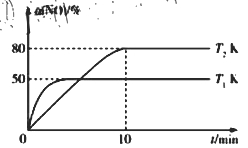
��Ŀ����
����Ŀ��������״���������г���84����Һ������������Ч�ɷ�ΪNaClO��Һ������Ҫ�ǻ��ڴ����ᣨHClO���������ԡ������ڵ�±��Ԫ���������������й㷺Ӧ�ã��ش��������⣺
��1����̬��ԭ�ӵĺ���۵����Ų�ʽΪ__________��HClO����������ԭ�ӵ��ӻ��������Ϊ__________��
��2������ũҩ��ԭ��PSCl3�У�P��S��Cl�ĵ縺���ɴ�С��˳��Ϊ_________��
��3������Cl���ڵ�Ԫ��S��F���仯����SF6���㷺������ѹ�����豸�ľ�Ե���ʡ�SF6��һ�ֹ��ۻ������ͨ��������Born��Haberѭ��������������ͼ��a��������ؼ��ܣ���S��F���ļ���Ϊ__________��
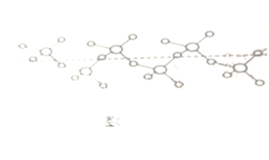
��������γɵ���״������ṹ��ͼ��C�����仯ѧʽΪ__________��
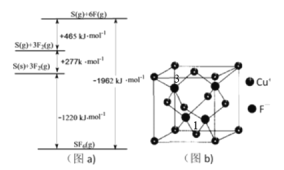
��4��CuCl���۵�Ϊ426�棬�ۻ�ʱ���������磻CuF���۵�Ϊ908�档
��CuF���۵��CuCl�ĸߣ�ԭ����_________��
�ڹ�ҵ�Ͻ�CuCl����KCN��Һ�����Ƴɶ�ͭҺ����ͭҺ������ﻯѧʽΪ__________��д��һ�������廥Ϊ�ȵ�����������ӵĵ���ʽ__________��
��CuF������ͼ��b���������߳�Ϊa nm����Cu����F������ľ���Ϊ________����Mg��mol��1��ʾCuF��Ħ��������NA��ʾ�����ӵ�������ֵ����CuF������ܶ�Ϊ______g��cm��3��
���Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������꣬����ͼ��b��������1������Ϊ��![]() ��
��![]() ��0����������3������Ϊ__________��
��0����������3������Ϊ__________��
���𰸡�3s23p5 sp3 Cl��S��P 327kJmol-1 (SO3)n CuCl�Ƿ��Ӿ��壬CuF�����Ӿ��� K3Cu(CN)4 ![]()
![]() nm
nm ![]()
![]()
��������
��1����̬��ԭ�����������Ӳ㣬����������Ϊ7���������۵����Ų�ʽΪ3s23p5��HClO���ӵ�����ԭ��Ϊ��ԭ�ӣ������ӻ�������ۣ����ӻ��������Ϊ sp3��
��2��ͬ����Ԫ�ش����ң��縺��Խ��Խǿ����P��S��Cl�ĵ縺���ɴ�С��˳��ΪCl��S��P��
��3������ͼ��a����֪6F(g)+S(g)=SF6(g) H=-1962 kJ/mol����S��F���ļ���Ϊ1962 kJ/mol��6��327kJmol-1��
����ͼ��C����֪��������γɵ���״���������ɶ��SO3���ӹ��ɵĽṹ�����仯ѧʽΪ(SO3)n��
��4������ΪCuF�����Ӿ��壬CuCl�Ƿ��Ӿ��壬��CuF���۵��CuCl�ĸߡ�
�ڽ�CuCl����KCN��Һ���γ�����Cu+�ṩ�չ�������������ӣ���λ��Ϊ4�����仯ѧʽΪK3Cu(CN)4�������廥Ϊ�ȵ�����������ӿ���ΪNO+������ʽΪ![]() ��
��
����ͼ��b����ʾ�������߳�Ϊa nm�������Խ��߳���Ϊ![]() nm��������Cu����F������ľ���Ϊ��Խ��ߵ�
nm��������Cu����F������ľ���Ϊ��Խ��ߵ�![]() ������Ϊ
������Ϊ![]() nm����������4��Cu����F���������ܶȹ�ʽ����֪
nm����������4��Cu����F���������ܶȹ�ʽ����֪![]() ��
��
�ܽ��þ����г�8�ȷݣ���8��С�����壬����3λ�����Ϸ�����������ģ���������1������Ϊ��![]() ��
��![]() ��0����������3������Ϊ
��0����������3������Ϊ![]() ��
��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�