��Ŀ����
����Ŀ��1��3������ϩA�����ںϳ�CR��ҽҩ�м���K�����кϳ�K����·���£�

��֪��
I.�ȴ���D����Է���������113���ȵ���������ԼΪ62.8%���˴Ź����������֮��Ϊ2��1��
��. R-CH2COOH
R-CH2COOH
��1��B�������Ĺ�������__________��E��ϵͳ������������__________��
��2����Ӧ�ڵ�������__________������д���ٺ͢۵ķ�Ӧ����__________��__________��
��3��д��G��H�����з�Ӧ�Ļ�ѧ����ʽ________��
��4��K�Ľṹ��ʽΪ__________��
��5��д����G��2��̼ԭ�ӵ�ͬϵ�������ͬ���칹��Ľṹ��ʽ__________��
��6����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�CR��![]() ����·����
����·����
�����������̣����������ߺϳɹ���__________��
���𰸡���ԭ�ӡ�̼̼˫�� 1��3-������ O2��Cu(��Ag)�������� �ӳɷ�Ӧ ȡ����Ӧ HOOCCH2COOH+2CH3CH2OH![]() CH3CH2OOCCH2COOCH2CH3+2H2O
CH3CH2OOCCH2COOCH2CH3+2H2O ![]()

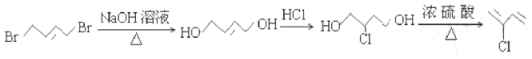
��������
1��3������ϩAͨ���ӳɷ�Ӧ�����л���C��ͨ����֪��������֪��C��H�ķ�Ӧ��������֪�����ͬ������H�ķ���ʽ��֪��ṹ��ʽΪH5C2OOCCH2COOC2H5�����ݷ�Ӧ��������֪G���Ҵ�����������Ӧ��G�Ľṹ��ʽΪHOOCCH2COOH��F��������Һ��Ӧ����G����F�к�ȩ����F�ṹ��ʽΪOHCCH2CHO�����ݷ�Ӧ��������֪I��֪��D����±������ȡ����Ӧ����E��E�����ǻ�����Ϊ�ȴ���D����Է���������113���ȵ���������ԼΪ62.8%�������һ��D�����к���������ԭ�ӣ�����˴Ź����������֮��Ϊ2��1����D�ṹ��ʽΪClCH2CH2CH2Cl��
��1������B�Ľṹ��ʽ��֪��B�����Ĺ���������ԭ�ӡ�̼̼˫����E�Ľṹ��ʽΪHOCH2CH2CH2OH����ϵͳ������������1��3-��������
��2����Ӧ��Ϊ���Ĵ���������ȩ����������O2��Cu(��Ag)�������������ݷ�Ӧǰ������ʽṹ��ʽ��֪����Ӧ��Ϊ�ӳɷ�Ӧ����Ӧ��Ϊȡ����Ӧ��
��3��G�Ľṹ��ʽΪHOOCCH2COOH���Ҵ�����������Ӧ��G�к��������Ȼ����ʷ�Ӧ�Ļ�ѧ����ʽΪHOOCCH2COOH+2CH3CH2OH![]() CH3CH2OOCCH2COOCH2CH3+2H2O��
CH3CH2OOCCH2COOCH2CH3+2H2O��
��4��������֪��![]() ��Ӧ����K�Ļ���������ͬ����K�ṹ��ʽΪ
��Ӧ����K�Ļ���������ͬ����K�ṹ��ʽΪ![]() ��
��
��5��G�Ľṹ��ʽΪHOOCCH2COOH����G��2��̼ԭ�ӵ�ͬϵ��������-COOH������ʽΪC5H8O4�����еĽṹ��ʽ�У� ��
��
��6��Ҫ�ϳ�CR��![]() �ۺ����ͨ��
�ۺ����ͨ��![]() ���мӾ۷�Ӧ�Ʊ�����B�ϳ�
���мӾ۷�Ӧ�Ʊ�����B�ϳ�![]() ������ͨ��±������ȡ������
������ͨ��±������ȡ������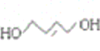 ����ͨ���ӳɷ�Ӧ����
����ͨ���ӳɷ�Ӧ����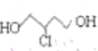 �����ͨ����ȥ��Ӧ�õ�
�����ͨ����ȥ��Ӧ�õ�![]() �������ߵĺϳɹ���Ϊ��
�������ߵĺϳɹ���Ϊ��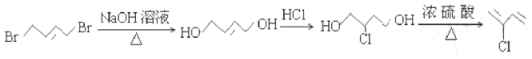 ��
��

����Ŀ�����ʡ�ī��ֽ���������й���ͳ�Ļ��б���Ϊ���ķ��ı���������˵���д�����ǣ� ��
A | B | C | D |
|
|
|
|
�����շ��ɼ������������������α | ī����Ҫ�ɷ���̼���� | ֽ����Ҫ�ɷ����ںϳɲ��� | ��ʯ��������̨�Ĺ����������仯 |
A.AB.BC.CD.D