��Ŀ����
����Ŀ��ͭ����������ʹ�õĽ���֮һ���䵥�ʼ���������й㷺����;��
��1����̬ͭԭ�Ӻ�����________�������෴�ĵ��ӡ�
��2����ͭ��ͭ������Ǧ��Ԫ�ذ�һ�������������ɵĺϽ𡣵�һ������I1(Sn)____________I1(Pb)(����ڡ���С�ڡ�)��
��3�����Ƶ�Cu(OH)2�ܹ��ܽ���Ũ��ˮ�У���Ӧ�����ӷ���ʽ��____________________________________��
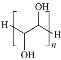
��4������ͭƬ�������Ӧ���ҹ��о���Ա����Ȳ����Ϊԭ�ϣ����������״�ͨ����ѧ�������ȫ̼���ϡ�ʯīȲ��Ĥ(�ṹƬ����ͼ��ʾ)���������˹���ѧ�ϳ�̼ͬ���������������ʯīȲ��̼ԭ��_________________________���ӻ���ʽ��

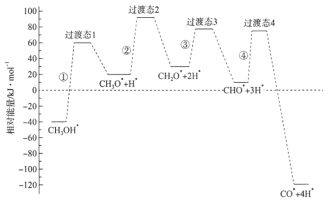
��5��CuCl��������Һ������CO�γ��Ȼ��ʻ���ͭ��I���������ڶ����ⶨ����������CO�ĺ������Ȼ��ʻ���ͭ��I���к�___________������Ŀ��
��6��Cu2O�����ڰ뵼����ϡ�
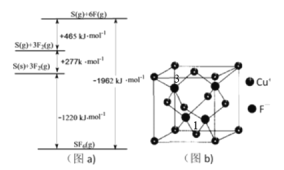
��Cu2O����(��ͼ��ʾ)�У�Oԭ�ӵ���λ��Ϊ________________��aλ��Cu+����Ϊ(0.25��0.25��0.75)����bλ��Cu+����_______________________��
��Cu2S��Cu2O�������ƾ���ṹ�������ߵ��۵���Cu2O��Cu2S��_________(��ߡ��͡�)�������ԭ��___________________��
���𰸡�14 ���� Cu(OH)2+4NH3��[Cu(NH3)4]2++2OH�� sp��sp2 14 4 (0.75��0.75��0.75) �� ���߶������Ӿ��壬O2���뾶��S2���뾶С������Cu2O�ľ����ܸ����۵����
��������
(1)CuΪ29��Ԫ�أ����ݹ���ԭ��֪���̬�����Ų�Ϊ1s22s22p63s23p63d104s1�����̬ͭԭ�Ӻ�����14�������෴�ĵ��ӣ�
(2)������Խǿ��Խ��ʧ���ӣ���һ������ԽС��Sn��PbΪͬ����Ԫ�أ��ҽ�����SnС��Pb�����һ������I1(Sn)����I1(Pb)��
(3)���Ƶ�Cu(OH)2�ܹ��ܽ���Ũ��ˮ�У����ɿ�����ˮ��[Cu(NH3)4]2+����Ӧ�����ӷ���ʽ��Cu(OH)2+4NH3��[Cu(NH3)4]2++2OH����
(4)̼̼������Cԭ�Ӽ۲���ӶԸ�����2�Ҳ����µ��Ӷԣ������ӻ���ʽΪsp�������е�̼�γ�3���������µ��Ӷԣ���ȡsp2�ӻ���
(5)���Ȼ��ʻ���ͭ(I)��C��O֮����1��������H2O��������2����������λ��Ҳ�����������Ȼ��ʻ���ͭ(I)�к�14��������
(6)��Cu2O�����У�Oԭ����Χ�����Cuԭ����Ŀ��4����Oԭ�ӵ���λ��Ϊ4��aλ��Ϊ����Oԭ�Ӻ�����Oԭ�ӵ�![]() �����Ҷ���Oԭ����a֮��ľ���Ϊ�Խ��ߵ�
�����Ҷ���Oԭ����a֮��ľ���Ϊ�Խ��ߵ�![]() ����֪aλ��Cu+����Ϊ(0.25��0.25��0.75)���������߳�Ϊ1����bλ��Cu+����Ϊ(0.75��0.75��0.75)��
����֪aλ��Cu+����Ϊ(0.25��0.25��0.75)���������߳�Ϊ1����bλ��Cu+����Ϊ(0.75��0.75��0.75)��
��Cu2S��Cu2O�������Ӿ��壬O2���뾶��S2���뾶С������Cu2O�ľ����ܸ�����Cu2O�۵���ߡ�

 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�����Ŀ���¶�ΪT1ʱ�����ݻ�Ϊ2 L ���ܱ������ס����зֱ����һ������CO(g)��H2O(g)��������Ӧ��CO(g) + H2O(g) ![]() CO2(g) + H2(g) H = ��41 kJ/mol���������£�����˵������ȷ����
CO2(g) + H2(g) H = ��41 kJ/mol���������£�����˵������ȷ����
���� | �� | �� | ||
��Ӧ�� | CO | H2O | CO | H2O |
��ʼʱ���ʵ�����mol�� | 1.2 | 0.6 | 2.4 | 1.2 |
ƽ��ʱ���ʵ�����mol�� | 0.8 | 0.2 | a | b |
A. �������У�ƽ��ʱ����Ӧ�ų�������Ϊ16.4 kJ
B. T1ʱ����Ӧ��ƽ�ⳣ��K�� = 1
C. ƽ��ʱ������CO��Ũ���Ǽ��е�2��
D. �������У�ƽ��ʱCO��ת����ԼΪ75%