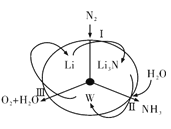
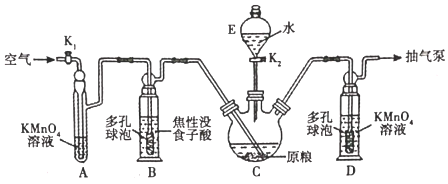
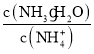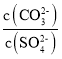��Ŀ����
����Ŀ��Q��R��X��Y��ZΪǰ20��Ԫ���е����֣�Q�ĵͼ���������X���ʷ��ӵĵ���������ȣ�R��Qͬ�壬Y��Z��������Arԭ�ӵĵ��ӽṹ��ͬ��Y��ԭ������С��Z��
(1)Q�������������̬���� ���壬������ ��
(2)R���⻯����ӵĿռ乹���� ������ ����(���������������Ǽ�����)������X�γɵĻ��������Ϊһ����Ҫ���մɲ��ϣ��仯ѧʽ�� ��
(3)X�ij����⻯��Ŀռ乹���� ��������һ�⻯��X2H4��һ�ֻ��ȼ�ϵijɷ֣������ʽ�� ��
(4)Q�ֱ���Y��Z�γɵĹ��ۻ�����Ļ�ѧʽ�� �� ��Q��Y�γɵķ��ӵĵ���ʽ�� ������ ����(���������������Ǽ�����)��
���𰸡���1������ �ɱ�
��2���������� �Ǽ��� Si3N4
��3��������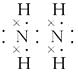
��4��CS2CCl4![]() �Ǽ���
�Ǽ���
��������
�����ۺϿ������ʽṹ֪ʶ������������������֪QΪC��RΪSi��XΪN��YΪS��ZΪCl��
��1��C�����������ΪCO2����̬ʱ�Ƿ��Ӿ��壬������Ϊ�ɱ���
��2��Si���⻯��ΪSiH4������CH4�Ŀռ乹�Ϳ�֪SiH4�Ŀռ乹��Ϊ�������壬SiH4������������������غϣ����ڷǼ��Է��ӡ�Si�������4�����ӣ�N�������5�����ӣ������γɻ�����ʱ��SiΪ��4�ۣ�NΪ��3�ۣ��仯ѧʽΪSi3N4��
��3��N�ij����⻯��ΪNH3����ռ乹��Ϊ�����Ρ�����N��Hԭ�ӵijɼ�ԭ�ɵ�N2H4�Ľṹ��ʽΪH2N��NH2��������ṹ��ʽ����д�������ʽ��
��4��C��S���γ�CS2������CO2�Ľṹ��д��CS2�ĵ���ʽ��֪��Ϊ�Ǽ��Է��ӡ�C��Cl�γ�CCl4���ӡ�

����Ŀ��Ӱ�컯ѧ��Ӧ���ʵ����غܶ�,ij������ȤС����ʵ��ķ�������̽����
ʵ��һ:ȡ�����ʵ���Ũ�ȵ����H2O2��Һ�ֱ��������ʵ��,ʵ�鱨�����±���ʾ��

��� | ���� | ���� | ���� | |
�¶�/�� | ���� | |||
1 | 40 | FeCl3��Һ | ||
2 | 20 | FeCl3��Һ | ||
3 | 20 | MnO2 | ||
4 | 20 | �� | ||
��1��ʵ��1��2��Ŀ�����о�__________���ض�H2O2�ֽ����ʵ�Ӱ�졣
��2��ʵ��1��Ӧ�Ļ�ѧ����ʽΪ___________________________��
ʵ���:���о�֪Cu2+��H2O2�ֽ�Ҳ���д�����,Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч��,��С���ͬѧ�ֱ��������ͼ��ʾ��ʵ�顣��ͨ���۲�_________�ó����ۡ� ��ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ����,��������____________________��
ʵ����:��֪�ڸ�����أ�KMnO4��������Һ�Ͳ��ᣨH2C2O4����Һ��Ӧʱ,���ֿ�ʼһ��ʱ��,��Ӧ���ʽ���,��Һ��ɫ�����ԣ�������ͻȻ��ɫ,��Ӧ�������Լӿ졣
��1��д�������ữ�ĸ��������Һ�Ͳ�����Һ��Ӧ�Ļ�ѧ����ʽ____________
��2���������ʵ������,ijͬѧ��ΪKMnO4��H2C2O4��Ӧ�Ƿ��ȷ�Ӧ,������Һ�¶�����,��Ӧ���ʼӿ졣��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�,��IJ��뻹������______��
��3������ʵ��֤����IJ���/span>,�����Ը��������Һ��������Һ�Լ���,����Ҫѡ����Լ����������__________��
A������� B�������� C��ˮ D���Ȼ���
��4����ʵ������н�2.0mL0.10mol/LH2C2O4��Һ��4.0mL0.010mol/L����KMnO4��Һ��ϣ�������Һ��Ϻ�����ı仯���������Һ����ɫʱ��Ϊ40s,���ʱ����ƽ����Ӧ����v(KMnO4)=__________mol��L-1��min-1��