��Ŀ����
��14�֣�ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ��������
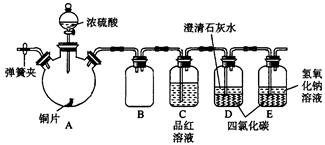
ʵ��ѡ��ͭƬ��98.3%��H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭƬһ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
�ش��������⡣
��1��Cu��ŨH2SO4�ķ�Ӧ�Ļ�ѧ����ʽΪ____________________��
��2��D��E��������CCl4��������____________________��
��3�����ȹ����У��۲쵽A�����г��ִ�����ɫ���������ŷ�Ӧ�Ľ��У�A�������а�ɫ�������ɣ�����Ϊ�ó�������_________���������ܵ�ԭ����________________________________________��
��4����A�����е�ŨH2SO4��ͭƬ���м��ȣ��ܿ췢��C������Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ�����_________________________________________�����ʵ����֤��IJ���_______________________________________��
��5��ʵ�������Ϊ�˼��ٻ�����Ⱦ���ų���װ���е�SO2���ɲ�ȡ�IJ�����________________________________________________________��

ʵ��ѡ��ͭƬ��98.3%��H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭƬһ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
�ش��������⡣
��1��Cu��ŨH2SO4�ķ�Ӧ�Ļ�ѧ����ʽΪ____________________��
��2��D��E��������CCl4��������____________________��
��3�����ȹ����У��۲쵽A�����г��ִ�����ɫ���������ŷ�Ӧ�Ľ��У�A�������а�ɫ�������ɣ�����Ϊ�ó�������_________���������ܵ�ԭ����________________________________________��
��4����A�����е�ŨH2SO4��ͭƬ���м��ȣ��ܿ췢��C������Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ�����_________________________________________�����ʵ����֤��IJ���_______________________________________��
��5��ʵ�������Ϊ�˼��ٻ�����Ⱦ���ų���װ���е�SO2���ɲ�ȡ�IJ�����________________________________________________________��
��1�� Cu+2H2SO4��Ũ��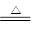 CuSO4+SO2��+H2O��2�֣� ��2����ֹ������2�֣�
CuSO4+SO2��+H2O��2�֣� ��2����ֹ������2�֣�
��3��CuSO4��2�֣���Ũ�����к�ˮ�٣����ɵ�����ͭ�϶࣬Ũ�������ˮ���ã�2�֣�
��4������SO2�ܽ�Ƚϴ���ʯ��ˮ��Ca��OH��2�����ͣ�������Ca��HSO3��2��Һ��Ե�ʣ�2�֣���ȡ���������м�������������Һ���۲��Ƿ��г������ɣ�2�֣��������ȡ����������SO2����ȷ�����
��5����A�����ϵĵ��ɼУ�����ͨ���������װ���е�SO2�ϵ�E�У��������B�м���NaOH��Һ�������ӣ����ɣ�2�֣���ע��ֱ����A�м�NaOH��Һ�����֣�
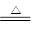 CuSO4+SO2��+H2O��2�֣� ��2����ֹ������2�֣�
CuSO4+SO2��+H2O��2�֣� ��2����ֹ������2�֣���3��CuSO4��2�֣���Ũ�����к�ˮ�٣����ɵ�����ͭ�϶࣬Ũ�������ˮ���ã�2�֣�
��4������SO2�ܽ�Ƚϴ���ʯ��ˮ��Ca��OH��2�����ͣ�������Ca��HSO3��2��Һ��Ե�ʣ�2�֣���ȡ���������м�������������Һ���۲��Ƿ��г������ɣ�2�֣��������ȡ����������SO2����ȷ�����
��5����A�����ϵĵ��ɼУ�����ͨ���������װ���е�SO2�ϵ�E�У��������B�м���NaOH��Һ�������ӣ����ɣ�2�֣���ע��ֱ����A�м�NaOH��Һ�����֣�
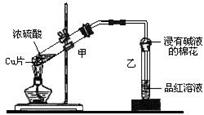
��1��Ũ�������ǿ�����ԣ���ͭ�ķ�Ӧ�ķ���ʽ��Cu+2H2SO4��Ũ��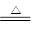 CuSO4+SO2��+H2O��
CuSO4+SO2��+H2O��
��2������SO2������ˮ�����ֱ��ͨ��SO2����������������SO2���������Ȼ�̼�������Ȼ�̼������ˮ���ܶȴ���ˮ���������Ȼ�̼�����þ��Ƿ�ֹ������
��3������Ũ�����к��е��ܼ�ˮ���٣������ɵ�����ͭ�϶࣬��Ũ���������ˮ�ԣ����Ի�������ͭ����������
��4������SO2�ܽ�Ƚϴ���ʯ��ˮ��Ca��OH��2�����ͣ����SO2�ǹ����ģ����Բ�����������Ƴ��������������˿����Ե�Ca��HSO3��2��������ڲ�����ǡ�����Ca��HSO3��2�ܺ�ǿ�Ӧ���ɳ������ݴ˿��Լ��𡣻�������Ca��HSO3��2�����ᷴӦ����SO2�����顣��ȡ���������м�������������Һ���۲��Ƿ��г������ɣ������ȡ����������SO2����ȷ�������
��5������װ���л����û�лӷ�����SO2��������ȷ�������Ǵ�A�����ϵĵ��ɼУ�����ͨ���������װ���е�SO2�ϵ�E�У��������B�м���NaOH��Һ�������ӣ����ɡ�
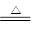 CuSO4+SO2��+H2O��
CuSO4+SO2��+H2O����2������SO2������ˮ�����ֱ��ͨ��SO2����������������SO2���������Ȼ�̼�������Ȼ�̼������ˮ���ܶȴ���ˮ���������Ȼ�̼�����þ��Ƿ�ֹ������
��3������Ũ�����к��е��ܼ�ˮ���٣������ɵ�����ͭ�϶࣬��Ũ���������ˮ�ԣ����Ի�������ͭ����������
��4������SO2�ܽ�Ƚϴ���ʯ��ˮ��Ca��OH��2�����ͣ����SO2�ǹ����ģ����Բ�����������Ƴ��������������˿����Ե�Ca��HSO3��2��������ڲ�����ǡ�����Ca��HSO3��2�ܺ�ǿ�Ӧ���ɳ������ݴ˿��Լ��𡣻�������Ca��HSO3��2�����ᷴӦ����SO2�����顣��ȡ���������м�������������Һ���۲��Ƿ��г������ɣ������ȡ����������SO2����ȷ�������
��5������װ���л����û�лӷ�����SO2��������ȷ�������Ǵ�A�����ϵĵ��ɼУ�����ͨ���������װ���е�SO2�ϵ�E�У��������B�м���NaOH��Һ�������ӣ����ɡ�

��ϰ��ϵ�д�
 Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ
 Fe2O3+SO2��+ SO3��+ 14H2O
Fe2O3+SO2��+ SO3��+ 14H2O