��Ŀ����
7��ij��ѧʵ��С��ͬѧ��������װ���Ʊ���������̽�����������ʣ�������������ȥ����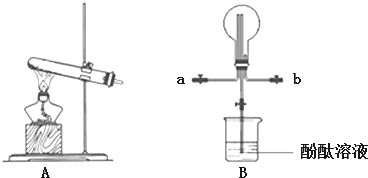
��1��ʵ�����Ʊ������Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+CaCl2+2H2O��
��2���ռ�����ʱ������ѡ�������a���a����b������
��3�����۲쵽װ��B�е���ƿ�ڲ����˺�ɫ��Ȫ����˵���������е������Ǽ�������ˮ����ˮ��Ӧ���ɼ
��4�������ڴ������Ҽ���ʱ�ᱻ�������������ǹ�ҵ������ĵ�һ����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��
��5���������İ�������ͨ��FeCl3��Һ�У��۲쵽�������Dz������ɫ������
���� ��1��ʵ�������Ȼ�����������Ƽ��ȷ�Ӧ�Ʊ�������
��2�������ܶ�С�ڿ����ܶȣ�Ӧѡ�������ſ������ռ���
��3��������Ȫʵ��ԭ�����
��4����������������һ��������ˮ��
��5��������ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ����Ȼ����������ֽⷴӦ���ɺ��ɫ��������������
��� �⣺��1���Ȼ�����������Ƽ��ȷ�Ӧ�����Ȼ��ơ�������ˮ����ѧ����ʽ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+CaCl2+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+CaCl2+2H2O��
��2�������ܶ�С�ڿ����ܶȣ�Ӧѡ�������ſ������ռ���Ӧ�����̳���
�ʴ�Ϊ��a��
��3��������������ˮ��ʹƿ��ѹǿѸ�ٽ��ͣ�������γɸ�ѹ�������Ȫ��
�ʴ�Ϊ����������ˮ����ˮ��Ӧ���ɼ
��4����������������һ��������ˮ����ѧ����ʽ��4NH3+5O2$\frac{\underline{\;������\;}}{\;}$4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{\;������\;}}{\;}$4NO+6H2O��
��5��������ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ����Ȼ����������ֽⷴӦ���ɺ��ɫ�����������������Իῴ���������ɫ������
�ʴ�Ϊ���������ɫ������
���� ���⿼���˰�����ʵ�����Ʊ������ʵļ��飬��Ϥ�Ʊ�ԭ���Ͱ��������ǽ���ؼ�����Ŀ�ѶȲ���

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�| A�� | V��O2 ��=0.01mol•L-1•s-1 | B�� | V��NO ��=0.08mol•L-1•s-1 | ||
| C�� | V��H2O��=0.01mol•L-1•s-1 | D�� | V��NH3��=0.002mol•L-1•s-1 |
| A�� | ����ˮ�ķ�Ӧ������ˮ�ķ�Ӧ������ | |
| B�� | �嵥����ˮ�ķ�Ӧ���ȵ�����ˮ�ķ�Ӧ������ | |
| C�� | ��˵���������ӣ������Ԫ�غ�±�ص�ԭ�Ӱ뾶�������� | |
| D�� | �����Ԫ���У��ԭ��ʧȥ�������ӵ��������� |
| A�� | ���ʵ���Ũ��Ϊ0.5mol•L-1��MgCl2��Һ�У�����Cl-����Ϊ1 NA | |
| B�� | ��״���£�11.2 L S03���еķ�����Ϊ0.5NA | |
| C�� | 1 mol Na2O2��������Ϊ4NA | |
| D�� | 24 g O2���Ӻ�24 g O3������������ԭ����Ŀ��� |
| A�� | Ũ���� | B�� | ϡ���� | C�� | Ũ���� | D�� | Ũ���� |
| A�� | ��״���£�4.4g CO2�к��е�ԭ����Ϊ0.3NA | |
| B�� | ��״���£�22.4L�����к��е�C-H����Ϊ4NA | |
| C�� | 5.6g�����������ᷴӦʧȥ�ĵ���������Ϊ0.25NA | |
| D�� | ��NA��Na+��Na2O�ܽ���1Lˮ�У�Na+�����ʵ���Ũ��Ϊ1 mol•L-1 |
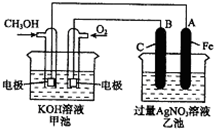 ��ͼ��һ����ѧ���̵�ʾ��ͼ��
��ͼ��һ����ѧ���̵�ʾ��ͼ��