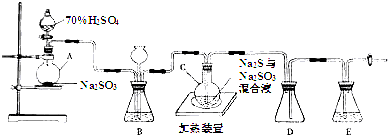
��Ŀ����
14�������а��־��壺A��ˮ����SiO2�� B�������� C������þ D������ E������� F���Ȼ�� G���� H�����ʯ������Żش��������⣺��1������ԭ�Ӿ���Ļ�������A��ֱ����ԭ�ӹ��ɵķ��Ӿ�����E��
��2�����й��ۼ������Ӿ�����F��ֻ�����Ӽ�����C��
��3����һ���������ܵ������������ѧ�����ѵ���G�������ۻ���ѧ���������仯�Ļ�������BDE�������ۻ�����˷����ۼ�����AH��
���� ��1��ԭ�Ӿ���Ĺ�����Ϊԭ�ӣ����Ӿ���Ĺ�����Ϊ���ӣ���ϡ�������γɵķ��Ӿ�����ԭ�ӹ��ɵķ��ӣ�
��2�����Ӿ�����һ���������Ӽ��������Ӿ����зǽ���Ԫ��֮���γɹ��ۼ������Ӿ�����ֻ��һ��Ԫ����ɵ�Ϊ���ʣ�
��3�������������������ѧ�����ѣ������ۻ���ѧ���������仯���Ƿ��Ӿ��壻ԭ�Ӿ����ۻ���˷����ۼ���
��� �⣺A��ˮ����SiO2���к����ۼ���Ϊ���ۻ����Ϊԭ�Ӿ��壻
B���������к����ۼ���Ϊ���ۻ����Ϊ���Ӿ��壻
C������þ�к����Ӽ���Ϊ���ӻ����Ϊ���Ӿ��壻
D�������к����ۼ���Ϊ���ʣ����ڷ��Ӿ��壻
E��������в�����ѧ����Ϊ���ʣ����ڷ��Ӿ��壻
F���Ȼ���к����Ӽ����ۼ���Ϊ���ӻ����Ϊ���Ӿ��壻
G����Ϊ�������ʣ�Ϊ�������壻
H�����ʯ�����ۼ���Ϊ���ʣ�����ԭ�Ӿ��壬
��1������ԭ�Ӿ���Ļ�������A��ֱ����ԭ�ӹ��ɵķ��Ӿ�����E���ʴ�Ϊ��A��E��
��2�����й��ۼ������Ӿ�����F��ֻ�����Ӽ�����C���ʴ�Ϊ��F��C��
��3����һ���������ܵ������������ѧ�����ѵ���G�������ۻ���ѧ���������仯�Ļ�������BDE�������ۻ�����˷����ۼ�����AH���ʴ�Ϊ��G��BDE��AH��
���� ���⿼�龧�����͵��жϼ���������ʣ�Ϊ��Ƶ���㣬ע�⾧��Ĺ���������ѧ�������ʵķ��༴�ɽ���ۺ��Խ�ǿ����Ŀ�ѶȲ���3��Ϊ�����ѵ㣮

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�| A�� | 200 | B�� | 770 | C�� | 290 | D�� | 292 |
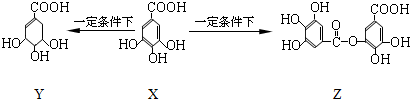
| A�� | 1 mol X�������2 mol Br2����ȡ����Ӧ | |
| B�� | Y���ӽṹ����3������̼ԭ�� | |
| C�� | Y�ܷ����ӳɡ�ȡ������ȥ������ | |
| D�� | 1 mol Z�������7 mol NaOH������Ӧ |
| A�� | NaCl | B�� | SiO2 | C�� | Ar | D�� | Na |
| A�� | ���Ӿ����е�ÿ��������һ�����й��ۼ� | |
| B�� | ����������۵�ͷе㶼�ܸ� | |
| C�� | ���Ӿ����п��ܺ��й��ۼ� | |
| D�� | ԭ�Ӿ����е�����ԭ�Ӽ�ֻ���ڷǼ��Թ��ۼ� |
| A�� | Ũ�������������ֽ⣬���뱣������ɫƿ�� | |
| B�� | ŨHNO3����ǿ����������ֻ��ʹʪ����ɫ��ʯ����ֽ�Ժ�ɫ������ɫ | |
| C�� | ϡHNO3�ͻ��ý�����Ӧʱ��Ҫ�õ����� | |
| D�� | �����£���ŨHNO3��Ͷ��FeƬ������������ĺ���ɫ���� |
���Ҵ��е�� ���Ҵ��ܶȱ�ˮС ���Ҵ��л�ԭ�� ���Ҵ������ĺ��������
| A�� | �ڢ� | B�� | �ڢ� | C�� | �٢� | D�� | �٢� |
| A�� | �����������������Na2CO3��Һ�������� | |
| B�� | ���飨C3H8��������ͬ���칹�� | |
| C�� | ��ϩ�ͱ������о�����̼̼˫�� | |
| D�� | ���ࡢ��֬�͵����ʾ��ɷ���ˮ�ⷴӦ |