��Ŀ����
����Ŀ���ߴ��辧������Ϣ��������Ҫ���ϡ�
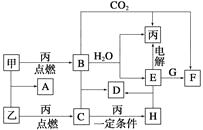
��1����ҵ����ʯӢ�ͽ�̿�����Ƶôֹ衣��֪��Ӧ���̵������仯����ͼ


д����ʯӢ�ͽ�̿��ȡ�ֹ���Ȼ�ѧ����ʽ______________________________��
��2��ijͬѧ������������Ʊ��ߴ��裺

��Y�Ļ�ѧʽΪ____________________��
��д����ӦI�����ӷ���ʽ________________________________________��
��д����ӦIV�Ļ�ѧ����ʽ________________________________________��
�ܼ���ֽ���¶�ԶԶ���ڹ��飨SiH4������ԭ�ӽṹ������ԭ��______________________��
��3�����ֹ�ת�������ȹ��飨SiHCl3������һ����ӦҲ�����Ƶôֹ衣�䷴Ӧ��SiHCl3(g)+H2(g)![]() Si(s)+3HCl(g)����ͬ�¶��£�SiHCl3��ƽ��ת�����淴Ӧ���Ͷ�ϱȵı仯��ϵ��ͼ��ʾ������˵����ȷ����__________������ĸ����
Si(s)+3HCl(g)����ͬ�¶��£�SiHCl3��ƽ��ת�����淴Ӧ���Ͷ�ϱȵı仯��ϵ��ͼ��ʾ������˵����ȷ����__________������ĸ����

A���÷�Ӧ�Ƿ��ȷ�Ӧ
B���������ʾ��Ͷ�ϱȿ�����![]()
C���÷�Ӧ��ƽ�ⳣ�����¶����߶�����
D��ʵ��������Ϊ���SiHCl3�������ʣ������ʵ�����ѹǿ
���𰸡� SiO2(s)+2C(s)=Si(s)+2CO(g) H=+638.4kJ/mol H2SiO3��H4SiO4 SiO2+2OH-=SiO32-+H2O SiO2+4Mg![]() Mg2Si+2MgO ���ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ���������ȶ������ڼ��� BC
Mg2Si+2MgO ���ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ���������ȶ������ڼ��� BC
����������1����Si(s)+O2(g)==SiO2(g) ��H=-859.4kl/mol����2C(s)+ O2(g)==2CO(g) ��H=-221.0kl/mol���ɢ�-�ٿɵ�ʯӢ�ͽ�̿��ȡ�ֹ���Ȼ�ѧ����ʽ��SiO2(s)+2C(s)=Si(s)+2CO(g) H=+638.4kJ/mol����2����XΪ�����ƣ���YΪ���ᣬ��д��H2SiO3��H4SiO4���ڷ�ӦI�����ӷ���ʽΪ��SiO2+2OH-=SiO32-+H2O����þ����������ڸ��������·�Ӧ�Ļ�ѧ����ʽΪ��SiO2+4Mg![]() Mg2Si+2MgO���ܼ���ֽ���¶�ԶԶ���ڹ��飨SiH4��Դ��̼����������ļ��ܴ�С�����ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ�̼����ļ��ܴ��ڹ�����ļ��ܣ���������ȶ������ڼ��顣��3����Ͷ�ϱ�һ��ʱʱ������Ͷ�ϱ����ߣ������¶����ߣ�T1����T2����T3����SiHCl3��ƽ��ת��������˵������Ӧ�����ȷ�Ӧ����A��������������Ũ�ȿ����SiHCl3��ƽ��ת���ʣ�B��ȷ��������Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����Ӧ��ƽ�ⳣ������C��ȷ�����ڷ�Ӧǰ���������С�ڷ�Ӧ�����������������ѹǿ��ƽ�������ƶ���SiHCl3��ƽ��ת���ʽ��ͣ�D����ѡBC��
Mg2Si+2MgO���ܼ���ֽ���¶�ԶԶ���ڹ��飨SiH4��Դ��̼����������ļ��ܴ�С�����ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ�̼����ļ��ܴ��ڹ�����ļ��ܣ���������ȶ������ڼ��顣��3����Ͷ�ϱ�һ��ʱʱ������Ͷ�ϱ����ߣ������¶����ߣ�T1����T2����T3����SiHCl3��ƽ��ת��������˵������Ӧ�����ȷ�Ӧ����A��������������Ũ�ȿ����SiHCl3��ƽ��ת���ʣ�B��ȷ��������Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����Ӧ��ƽ�ⳣ������C��ȷ�����ڷ�Ӧǰ���������С�ڷ�Ӧ�����������������ѹǿ��ƽ�������ƶ���SiHCl3��ƽ��ת���ʽ��ͣ�D����ѡBC��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
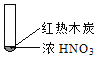
�����߿����ϵ�д�����Ŀ������ʵ���о��к���ɫ����������Աȷ������ý��۲���ȷ���ǣ�������
|
|
|
�� | �� | �� |
A. �����еĺ���ɫ���壬�ƶϲ���������һ���ǻ������
B. ����ɫ���岻�ܱ�������ľ̿��Ũ��������˷�Ӧ
C. ����˵��Ũ������лӷ��ԣ����ɵĺ���ɫ����Ϊ��ԭ����
D. ������������м���CO2���ɴ�˵��ľ̿һ����Ũ���ᷢ���˷�Ӧ
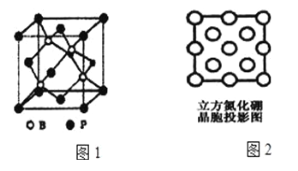
����Ŀ��X��Y��Z��M��Q��R��6�ֶ�����Ԫ�أ���ԭ�Ӱ뾶����Ҫ���ϼ����£�
Ԫ�ش��� | X | Y | Z | M | Q | R |
ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.089 | 0.074 | 0.037 |
��Ҫ���ϼ� | +2 | +3 | +6��-2 | +2 | -2 | +1 |
��1��Z��Ԫ�����ڱ��е�λ����______________________________��
��2��X��Y��Q�����γɼ����ӣ��������Ӱ뾶������__________�������ӷ��ţ���
��3�����ڱ�����Щ���ڶԽ��ߣ����ϡ����£�λ�õ�Ԫ�أ����ǵĵ��ʼ��仯������������ƣ���M����������ǿ����Һ��Ӧ�����ӷ���ʽ______________________________��
��4��Q��R��ԭ�Ӹ�����1:1��ɵĻ��������һ�֡���ɫ����������
�ټ����������ۼ�������____________________��
�ڿ�������������Ʊ���װ����ͼ��ʾ���ڼ�����Һ�У����ÿ����е�������ԭ�õ���ϡ�����Һ��ͼ��a��__________���������ĵ缫��Ӧʽ��____________________��

����Ŀ��ijС��������ͼװ�ã��ñ�������FeBr3���������Ʊ��屽��

�� | �� | �屽 | |
�ܶ�/g��cm-3 | 0.88 | 3.10 | 1.50 |
�е�/��C | 80 | 59 | 156 |
ˮ���ܽ��� | �� | �� | �� |
ʵ����̣���a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�塣��a�е��뼸���塣��Ӧ���ҽ��С���Ӧֹͣ���������̷����ᴿ��Ʒ��
 ��
��
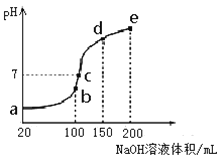
��1����ƿ���д�������ɫ�������Թ�d�е������ǣ���______________���� ����ˮ��ɻ�ɫ��c��������___________________________________��
��2�������ᴿʱ��������Ϊ______________��������Ϊ_________________��
��3����ˮϴ����Ҫ�õ��IJ���������_________���ձ�����ˮϴ��������ˮ���еμ�KSCN��Һ����Һ���ɫ���Ʋ�ˮϴ����ҪĿ���dz�ȥ__________________��
��4����NaOH��Һϴ��ʱ��Ӧ�Ļ�ѧ����ʽ��________________________��
��5����֪�����巢������ȡ����Ӧ���ƲⷴӦ���Թ�d��Һ�庬�е����ִ���������H+��Br�������ʵ�鷽����֤�Ʋ⡣����ѡ�Լ���Mg��CCl4��AgNO3aq��H2O��
ʵ�鲽�� | Ԥ������ | ���� |
����1�����Թ�d��Һ��ת���Һ©���� __________________________________������ȡ��Һ�ȷֳ����ݣ�����A��B���Թ��У����в���2��3�� | ||
����2�� �� | ֤���� ���� | |
����3�� �� | ֤���� ���� |