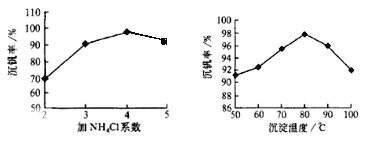
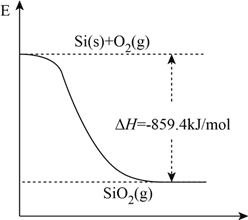
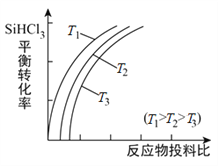
��Ŀ����
����Ŀ����������Ҫ��һԪ�ᣬ���л�������Ӧ�ж���Ӧ�á�����25 ��ʱ��pH��3�Ĵ��ᡣ��ش��������⣺
��1����������м������������ƹ��壬��ʱ��Һ��![]() ________(��������������С������������)��
________(��������������������������)��
��2����������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������Һ��pH________(����>����<����������)7�������ӷ���ʽ��ʾ��ԭ��_____________________________________��
��3����������м���pH��11��NaOH��Һ���Ҷ��ߵ������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����___________________________________________��
��4����������м���NaOH��Һ����Һǡ�ó����ԣ���ʱc(Na��)______c(CH3COO-)(����>������<����������)��
��5����������м���һ����NaOH��Һ�����û��ҺpH��6�������Һ��c(CH3COO-)��C(Na��)��________mol/L(��дȷ����)��
���𰸡� ��С > CH3COO����H2O![]() CH3COOH��OH�� cCH3COO��)>c(Na��)>c(H��)>c(OH��) �� 9.9��10��7
CH3COOH��OH�� cCH3COO��)>c(Na��)>c(H��)>c(OH��) �� 9.9��10��7
��������(1)������ƹ���������CH3COO-��c(CH3COO-)����ʹ��ƽ��CH3COOH![]() CH3COO-+H+�����ƶ���c(H+)��С��c(CH3COOH)��������
CH3COO-+H+�����ƶ���c(H+)��С��c(CH3COOH)��������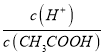 ��С���ʴ�Ϊ����С��
��С���ʴ�Ϊ����С��
(2)������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ�������˴����ƣ������ˮ��CH3COO-+H2O![]() CH3COOH+OH-����Һ�ʼ��ԣ���pH��7���ʴ�Ϊ������CH3COO-+H2O
CH3COOH+OH-����Һ�ʼ��ԣ���pH��7���ʴ�Ϊ������CH3COO-+H2O![]() CH3COOH+OH-��
CH3COOH+OH-��
(3)�����������Һ�еijɷ�Ϊ����ʹ����ƣ������ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ���Һ�����ԣ�����c(CH3COO-)��c(Na+)��c(H+)��c(OH-)���ʴ�Ϊ��c(CH3COO-)��c(Na+)��c(H+)��c(OH-)��
(4)Һǡ�ó����ԣ���c(H+)=c(OH-)�����ݵ���غ�c(Na+)+c(H+)=c(OH-)+c(CH3COO-)����c(Na+)=c(CH3COO-)���ʴ�Ϊ��=��
(5)pH=6��Һ�У�c(H+)=10-6mol/L��c(OH-)=10-8mol/L�����ݵ���غ�c(Na+)+c(H+) =c(OH-)+c(CH3COO-)��֪��c(CH3COO-)-c(Na+)=c(H+)-c(OH-) =10-6mol/L-10-8mol/L =(10-6-10-8)mol/L=9.9��10��7 mol/L���ʴ�Ϊ��9.9��10��7mol/L

 ��������ϵ�д�
��������ϵ�д�����Ŀ����CO��H2S��Ӧ���Ƶ��ʻ���COS�����ں��ݵ��ܱ������з�����Ӧ���ﵽƽ�⣺CO(g)+H2S(g) ![]() COS(g)+H2(g)���������±���ʾ��
COS(g)+H2(g)���������±���ʾ��
ʵ�� | �¶�/�� | ��ʼʱ | ƽ��ʱ | |||
n(CO)/mol | n(H2S)/mol | n(COS)/mol | n(H2)/mol | n(CO)/mol | ||
1 | 150 | 10.0 | 10.0 | 0 | 0 | 7.0 |
2 | 150 | 7.0 | 8.0 | 2.0 | 4.5 | a |
3 | 400 | 20.0 | 20.0 | 0 | 0 | 16.0 |
����˵����ȷ���ǣ� ��
A. ������Ӧ�����ȷ�Ӧ
B. ʵ��1��ƽ��ʱ��CO��ת����Ϊ70%
C. ʵ��2��ƽ��ʱ��a<7.0
D. ʵ��3��ƽƽ����ٳ���1.0molH2��ƽ�������ƶ���ƽ�ⳣ��ֵ����