��Ŀ����
10��A��һ�־��ô��ᵯ����Ҫ�ɷ֣�������A����Է�������Ϊ161��������C��HԪ���⣬��������һ��±��Ԫ�أ�������ֻ����һ������������A��H��ת����ϵ����ͼ��ʾ����������������Cu��OH��2����Һ��1mol C��Ӧ������1mol Cu2O ��1mol D��B1��B2��Ϊͬ���칹�壬B1��Ħ������80g/mol��G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1����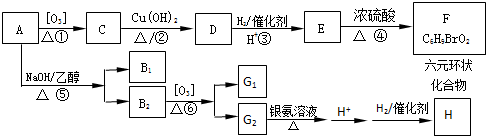
��֪����
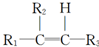 $\stackrel{[O_{3}]}{��}$
$\stackrel{[O_{3}]}{��}$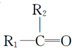 +
+
��һ��̼ԭ������������̼̼˫���Ľṹ��-C=C=C-�����ȶ���
�������������
��1��A�й����ŵ�������ԭ�Ӻ�̼̼˫��
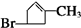
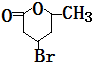
��2���١���Ӧ������ȡ����Ӧ���Ǣܣ�A�Ľṹ��ʽ��
��3����Ӧ�ܵĻ�ѧ����ʽ��HOOCCH2CHBrCH2CH��OH��CH3

 +H2O
+H2O��4��д��C������Cu��OH��2��Ӧ�ķ���ʽ��
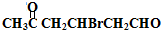 +2Cu��OH��2+NaOH$\stackrel{��}{��}$
+2Cu��OH��2+NaOH$\stackrel{��}{��}$ +Cu2O��+3H2O
+Cu2O��+3H2O��5��һ��������H�ܹ����ɸ߷��ӻ����д����Ӧ�ķ���ʽ
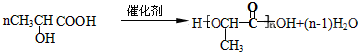
��6����������������E��ͬ���칹�干��2�֣�
�ٺ�����������������NaHCO3��Ӧ����-OH��-Br������ͬһ��̼ԭ���ϣ�
���� A��������F����6��Cԭ�ӡ�����1��Brԭ�ӣ���A�к���6��Cԭ�ӡ�1��Brԭ�ӣ�����ΪHԭ�ӣ�Hԭ�Ӹ���Ϊ161-80-6��12=9��A�ķ���ʽΪC6H9Br�������������IJ���ֻ��һ�֣���AӦ�ǻ�״���������ӽṹ��ֻ��һ��-CH3����AΪ��������Ԫ��״�����A�ij�������IJ���C��Cu��OH��2��Ӧ�ı�����ϵ֪C������ֻ��һ��ȩ������A�м��������е�һ��������̼ԭ���ϣ��ٽ����Ŀ���ṩ�Ľṹ��Ϣ֪��A�ṹΪ  ������������Ϣ��֪A��������������C��CΪ
������������Ϣ��֪A��������������C��CΪ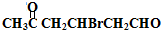 ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������ȡ������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������ȡ������Ӧ����F����FΪ ��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ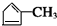 ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3���ݴ˽��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3���ݴ˽��
��� �⣺A��������F����6��Cԭ�ӡ�����1��Brԭ�ӣ���A�к���6��Cԭ�ӡ�1��Brԭ�ӣ�����ΪHԭ�ӣ�Hԭ�Ӹ���Ϊ161-80-6��12=9��A�ķ���ʽΪC6H9Br�������������IJ���ֻ��һ�֣���AӦ�ǻ�״���������ӽṹ��ֻ��һ��-CH3����AΪ��������Ԫ��״�����A�ij�������IJ���C��Cu��OH��2��Ӧ�ı�����ϵ֪C������ֻ��һ��ȩ������A�м��������е�һ��������̼ԭ���ϣ��ٽ����Ŀ���ṩ�Ľṹ��Ϣ֪��A�ṹΪ  ������������Ϣ��֪A��������������C��CΪ
������������Ϣ��֪A��������������C��CΪ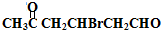 ��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ
��C����ΪD���������������ӳɷ�Ӧ�����������µõ�E����DΪ ��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������ȡ������Ӧ����F����FΪ
��EΪHOOCCH2CHBrCH2CH��OH��CH3��E����������ȡ������Ӧ����F����FΪ ��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ
��B1��A����ȥ��������ʽΪC6H8��B2��������õ�G1��G2��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1������B2Ϊ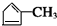 ��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3��
��G1ΪOHC-CH2-CHO��G2ΪOHCCOCH3����ת����ϵ��֪HΪHOOCCH��OH��CH3��
��1��������������֪��AΪ �����й�����Ϊ��ԭ�Ӻ�̼̼˫����
�����й�����Ϊ��ԭ�Ӻ�̼̼˫����
�ʴ�Ϊ����ԭ�Ӻ�̼̼˫����
��2��������������֪�����١���Ӧ������ȡ����Ӧ���Ǣܣ�AΪ ��
��
�ʴ�Ϊ���ܣ� ��
��
��3����Ӧ�ܵĻ�ѧ����ʽ��HOOCCH2CHBrCH2CH��OH��CH3
 +H2O��
+H2O��
�ʴ�Ϊ��HOOCCH2CHBrCH2CH��OH��CH3
 +H2O��
+H2O��
��4��CΪ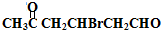 ��OHCCH2CHBrCH2COCH3 ������Cu��OH��2����Һ ��Ӧ�ķ���ʽΪ��
��OHCCH2CHBrCH2COCH3 ������Cu��OH��2����Һ ��Ӧ�ķ���ʽΪ��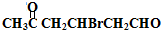 +2Cu��OH��2+NaOH$\stackrel{��}{��}$
+2Cu��OH��2+NaOH$\stackrel{��}{��}$ +Cu2O��+3H2O��
+Cu2O��+3H2O��
�ʴ�Ϊ��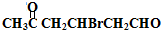 +2Cu��OH��2+NaOH$\stackrel{��}{��}$
+2Cu��OH��2+NaOH$\stackrel{��}{��}$ +Cu2O��+3H2O��
+Cu2O��+3H2O��
��5��H��һ�����������ɸ߾���ķ�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6��E��ͬ���칹������������ٺ�����������������NaHCO3 ��Ӧ������-COOH����-OH��-Br����ͬһ��̼ԭ���ϣ������������E��ͬ���칹��Ϊ��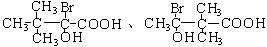 ����2�֣�
����2�֣�
�ʴ�Ϊ��2��
���� ���⿼���л�����ƶϺͺϳɣ���Ŀ��Ϊ�ۺϣ��ѶȽϴ�����ʱע������ú������Ϣ���������������ϵķ����ƶϣ���ѧ�����������нϸߵ�Ҫ��ȷ��A�Ľṹ�ǹؼ���ע��ͬ���칹����Ŀ���жϷ�����

| A�� | ������ˮ�м������NaOH���壬KW���� | |
| B�� | 0.1mol/LHF��Һ��PH=2�������Һ��c��OH-����c��HF�� | |
| C�� | ��Na2Sϡ��Һ�У�c��H+��=c��OH-��-2c��H2S��-c��HS-�� | |
| D�� | NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
| A�� | V��$\frac{1}{18}$ | B�� | V��$\frac{1}{23}$ | C�� | V��$\frac{1}{18}$ | D�� | V��$\frac{1}{56}$ |
| A�� | FeCl3��Һ�еμ�KSCN��Һ��Fe3++3SCN-�TFe��SCN��3�� | |
| B�� | ��������Һ��ͨ������CO2�� O-+CO2+H2O�� O-+CO2+H2O�� OH+HCO3- OH+HCO3- | |
| C�� | NaHCO3��Һ�мӹ���Ca��OH��2��Һ��Ca2++2HCO3-+2OH-�TCaCO3��+H2O+CO32- | |
| D�� | BaCO3�����м�������ϡH2SO4��BaCO3+2H+�TBa2++CO2��+H2O |
| A�� | NaOH�ĵ���ʽ�� | |
| B�� | ����������������Ϊ16����ԭ�ӣ�1616S | |
| C�� | ��ԭ�ӵĽṹʾ��ͼ��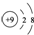 | |
| D�� | ��ȩ�Ľṹ��ʽ��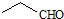 |
| A�� | ԭ�Ӱ뾶��Z��Y��W�������Ӱ뾶��W��Y��Z | |
| B�� | ��W������Һһ�������� | |
| C�� | ��X��Y��Z��ɵ��ε�ˮ��Һ�����ԣ�����Һ�и���������Ũ�ȴ����������Ũ�� | |
| D�� | W��Y�γɵ�ԭ�Ӹ�����Ϊ1��1�Ĺ��ۻ�����������¡�������������Ժõ��������ʣ����㷺Ӧ���ڵ��ӹ�ҵ���մɹ�ҵ��������һ��ԭ�Ӿ��� |
 ��
��
 ����������ѧ������Ϊ���ۼ������Ӽ���
����������ѧ������Ϊ���ۼ������Ӽ���